A new study unveils a precise picture of how an ion channel found in most mammalian cells regulates its own function with a “ball-and-chain” channel-plugging mechanism, according to investigators at Weill Cornell Medicine. The findings boost the understanding of ion channel biology and could lead to new drugs that target these channels to treat disorders such as epilepsy and hypertension.
Ion channels are protein structures embedded in cell membranes that allow charged molecules to flow into or out of the cell. They support essential biological functions, including signaling or communication between brain cells. The study, published Feb. 19 in Nature Communications, focused on the mammalian BK (“big potassium”) channel, which facilitates the flow of potassium ions out of cells.
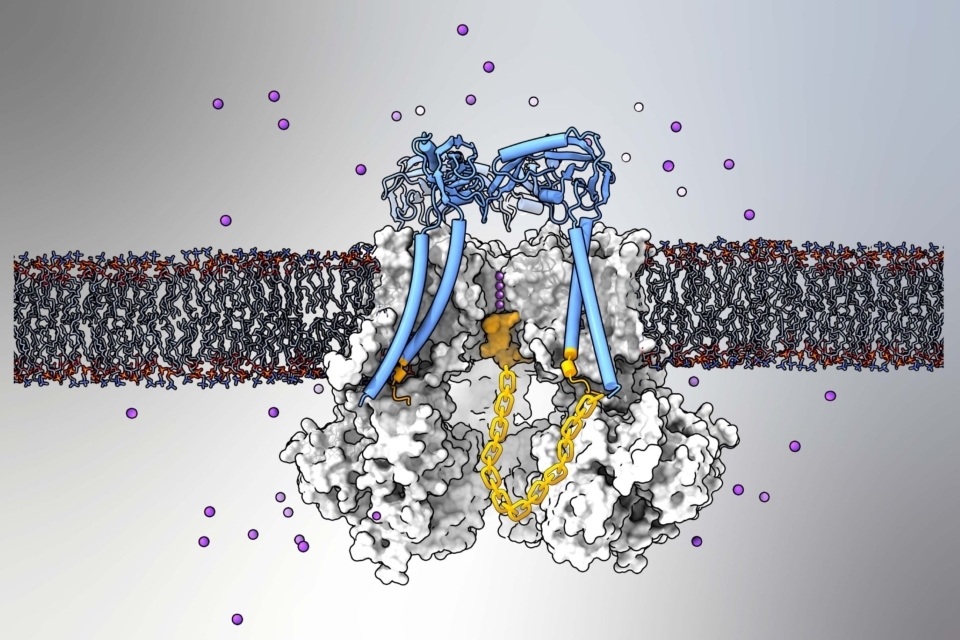
Using advanced structural imaging and computer modeling techniques, the researchers confirmed that BK channels can stop their ion flow via a long-theorized ball-and-chain structure that plugs the channel.

Dr. Crina Nimigean
“These findings provide insights into a fundamental mechanism in biology and point the way to better methods for modulating ion channel activity to treat human diseases,” said study senior author Dr. Crina Nimigean, a professor of physiology and biophysics in anesthesiology at Weill Cornell Medicine.
BK channels help govern the excitability of brain and muscle cells, control blood flow through blood vessels, process auditory signals and perform many other functions throughout the body. Accordingly, genetic and other dysfunctions of BK channels have been linked to a variety of disorders, from epilepsies and movement disorders to hypertension and hearing-loss syndromes. However, the complexity and fragility of BK channels have made studying them difficult.
Calcium ions can trigger a BK channel to open its central passage or “pore,” allowing a massive flow of potassium ions out of the cell. Research had long suggested—but never proven with direct imaging—that a BK channel can stop the flow through its calcium-triggered, open-pore using a ball-like plug that swings from the end of a flexible protein subunit.
In a widely cited study in 2020, Dr. Nimigean and colleagues revealed this ball-and-chain structure in a simpler potassium channel called MthK, an evolutionarily distant relative of BK channels found in bacterial organisms. In the new study, she and her team successfully identified this structure in Slo1, a more complex mammalian BK channel.

Dr. Alessio Accardi
The imaging work used low-temperature electron microscopy (cryo-EM) and required finding ways to stabilize the inherently loose and flexible channel structures. Dr. Nimigean and her lab collaborated with Dr. Alessio Accardi, professor of physiology and biophysics at Weill Cornell Medicine, who used computational modeling techniques to uncover the elusive structural details of how the protein plug blocks the pore.
“We couldn’t get a clear cryo-EM picture of this pore-binding structure because it binds in many different conformations,” Dr. Nimigean said. “Ultimately, with the help of the modeling, we found that the first three amino acids of the plug are very important for the binding, and the rest establishes a flexible chain length.”
Currently, Dr. Nimigean and her team are investigating how fat-related molecules in the cell membrane can influence BK channel activity also.
This work was sponsored in part by the National Institutes of Health grants GM088352, GM128420 and F32GM145091.