Neuroscientist Dr. Gary Gibson keeps a framed picture of a cell derived from the skin cells of a person with Alzheimer’s disease on his office wall.
The image is a memento of Dr. Gibson’s breakthrough hypothesis about an underlying cause of mild cognitive impairment and Alzheimer’s – that an insufficiency of vitamin B1 called thiamine alters the ability of mitochondria in brain cells to properly use glucose, directly causing neurodegeneration.
“It’s been well known since the 1930s that thiamine deficiency can cause dementia. However, in the late 90s, most other dementia researchers focused instead on the discoveries of amyloid plaques and tangles of tau protein, which were posited as potential causes of Alzheimer’s. They hypothesized that these abnormal brain changes caused lower glucose metabolism,” said Dr. Gibson, a professor of neuroscience in the Feil Family Brain and Mind Research Institute at Weill Cornell Medicine.
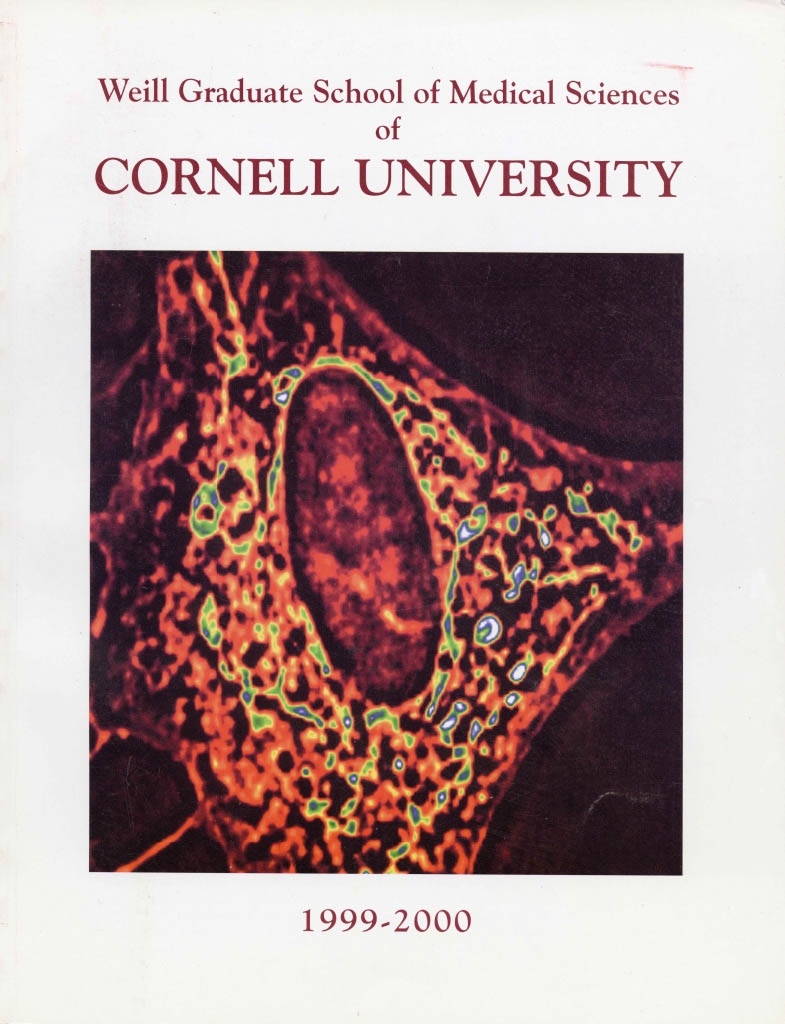
The same cell image appeared on the cover of Weill Cornell Graduate School of Medical Sciences' course catalogue in 1999.
“Our approach is just the opposite: We think thiamine deficiency alters glucose metabolism in brain cells, and that’s what leads to the formation of plaques and tangles,” he said. “Therefore, treating brain thiamine deficiency may be a better treatment target.”
Taken more than two decades ago, the image of the cell, or fibroblast, contains bright spots of color showing small, bean-shaped structures known as mitochondria, which transform glucose into cellular energy. He and his team found that mitochondria in fibroblasts derived from skin cells of Alzheimer’s patients showed abnormal glucose metabolism compared to those derived from individuals without the disease. The investigators subsequently converted the fibroblasts to neurons and found that the abnormal mitochondria lead to many Alzheimer’s-like changes. Determining why that was the case became the focus of Dr. Gibson’s research.
Dr. Gibson’s decades of hard work investigating this opposite approach have culminated in a nationwide clinical trial evaluating an entirely new treatment approach for slowing Alzheimer’s progression.
Early Research Findings Support Novel Hypothesis
Dr. Gibson’s early basic science work at the University of California, Los Angeles, in the late 1970s found that a common form of thiamine deficiency associated with neurodegeneration in people with alcohol use disorder interferes with glucose metabolism, and that thiamine requirements varied between individuals.
That connection was an attractive stepping stone for shifting his focus to studying the underlying biology of glucose metabolism and thiamine deficiency in Alzheimer’s. In the late 80s, he was recruited to join Weill Cornell Medicine and set up his lab at the Burke Neurological Institute, a Weill Cornell Medicine affiliate. He and his colleagues published numerous papers on findings that supported the hypothesis that altered glucose metabolism in brain cells caused by a thiamine deficiency could be a direct cause of Alzheimer’s disease.
Starting with a compound developed in Japan in the 1960s to address vitamin deficiencies in the general population, Dr. Gibson and colleagues focused on a synthetic oral drug, benfotiamine – a precursor or prodrug of thiamine.
“Benfotiamine boosts blood levels of thiamine to 100 times the normal levels, enabling more thiamine to move into brain cells to restore thiamine-dependent glucose utilization processes in mitochondria, and to diminish Alzheimer’s pathology,” said Dr. Gibson, who is also a professor of neurosciences at the Burke Neurological Institute and director of the Laboratory for Mitochondrial Biology and Metabolic Dysfunction in Neurodegeneration.
In preclinical research, benfotiamine successfully addressed the clinical and biological characteristics of Alzheimer’s, including impaired cognition, amyloid plaques, abnormal accumulations of tau protein called neurofibrillary tangles, lower glucose metabolism, oxidative stress and inflammation.
First-in-Human Pilot Study Results
In 2015, Dr. Gibson conducted a pilot clinical trial of benfotiamine at the Burke Rehabilitation Center to evaluate whether 12 months of treatment with the drug would enhance glucose utilization and minimize cognitive decline in patients with mild cognitive impairment or early Alzheimer’s. The study enrolled 70 patients.
The results were encouraging: Benfotiamine increased blood thiamine to high levels and showed strong signs of efficacy. For example, the increase in the Alzheimer’s Disease Assessment Scale-Cognitive Subscale was 43% lower in the benfotiamine group than in the placebo group, indicating less cognitive decline. Preliminary evidence from this study also showed that benfotiamine had promising potential for improving cognitive and functional outcomes.
A Larger Clinical Trial Opens
In May 2022, the National Institute of Aging, part of the National Institutes of Health, awarded the Burke Neurological Institute a $45 million grant to fund a larger clinical trial based on the strength of the trial proposal and evidence from the preclinical research and the pilot study. Dr. Gibson is working in collaboration with co-principal investigators Dr. Howard Feldman, director of the Alzheimer’s Disease Cooperative Study at the University of California San Diego, and Dr. Jose Luchsinger, vice-chair for Clinical and Epidemiological Research at Columbia University.
The phase 2A-2B study, called Benfoteam, will take place at 50 sites nationwide and seeks to enroll 406 patients ages 50 to 89 with mild cognitive impairment or mild dementia due to Alzheimer’s. The primary objectives are to determine the highest safe and well-tolerated dose of benfotiamine and whether it can improve global function and cognition over 18 months. The study began enrolling the first patients in April 2024, including at Weill Cornell Medicine and NewYork-Presbyterian/Weill Cornell Medical Center in September, with Dr. Anna Nordvig, assistant professor of neurology, serving as site PI.
The investigators will use advanced blood biomarker technologies for their study, in collaboration with groups around the world. They will work with the University of Cambridge, U.K. to assess thiamine levels; the University of Virginia to evaluate abnormal use of glucose; the University of Gothenburg, Sweden, to assess tau tangles, neuron loss and inflammation; and C2N Diagnostics, an American company, to evaluate amyloid plaques.
Dr. Gibson cautioned that taking over-the-counter vitamin B1 does not work as a strategy for raising brain thiamine levels, noting that the safest way for interested individuals to access benfotiamine is to participate in the trial. The study drug has been manufactured to meet specific standards. In contrast, benfotiamine sold online or from other sources is unregulated, not tested for safety and efficacy in clinical trials, and may not contain the ingredients indicated on the label.
“The recently approved drugs for treating Alzheimer’s, lecanemab and donanemab, are administered intravenously and work by removing amyloid plaques. They are relatively inconvenient, expensive and function to address disease symptoms,” Dr. Gibson said. “As an oral medication, benfotiamine is much more convenient, less expensive and, we believe, may do a much better job of targeting the root cause of the disease.”
“The fibroblast image on my office wall represents our immense effort. It also reminds me of all the people I worked with and our rigorous experiments testing what we thought was going to be important,” Dr. Gibson said. “Evidence-based science takes time. It’s truly exciting to watch our lab research translate into a clinical trial testing a new treatment for a disease that affects millions of patients.”
For more information about the trial and to locate a study site, visit ClinicalTrials.gov or benfoteam.org or call 877-807-1290 (toll-free).

