Ion channels are attractive drug targets due to their importance in health and disease, but finding ways to target a specific ion channel selectively is a major challenge. Now, researchers at Weill Cornell Medicine and RMIT University in Australia have discovered that ion channels called BK channels have unique openings in their sides, which drug molecules may be able to access. The finding, published Aug. 31 in Nature Chemical Biology, could lead to the development of selective drugs that target the BK channel to treat a wide range of diseases.
Ion channels are tunnel-like structures embedded in cell membranes that control the flow of charged molecules in or out of cells, which is required for many biological processes. BK channels, for instance, conduct the flow of potassium ions and inherited mutations in these channels have been linked to problems in multiple organ systems.
“The discovery of a site where small molecules can selectively access this important type of ion channel is an exciting development,” said study co-senior author Dr. Crina Nimigean, professor of physiology and biophysics in anesthesiology at Weill Cornell Medicine.
The other co-senior author of the study is Dr. Toby Allen, a professor at RMIT University in Melbourne, Australia. The first author, Dr. Chen Fan, was a postdoctoral research associate in the Nimigean Lab in the Department of Anesthesiology during the study.
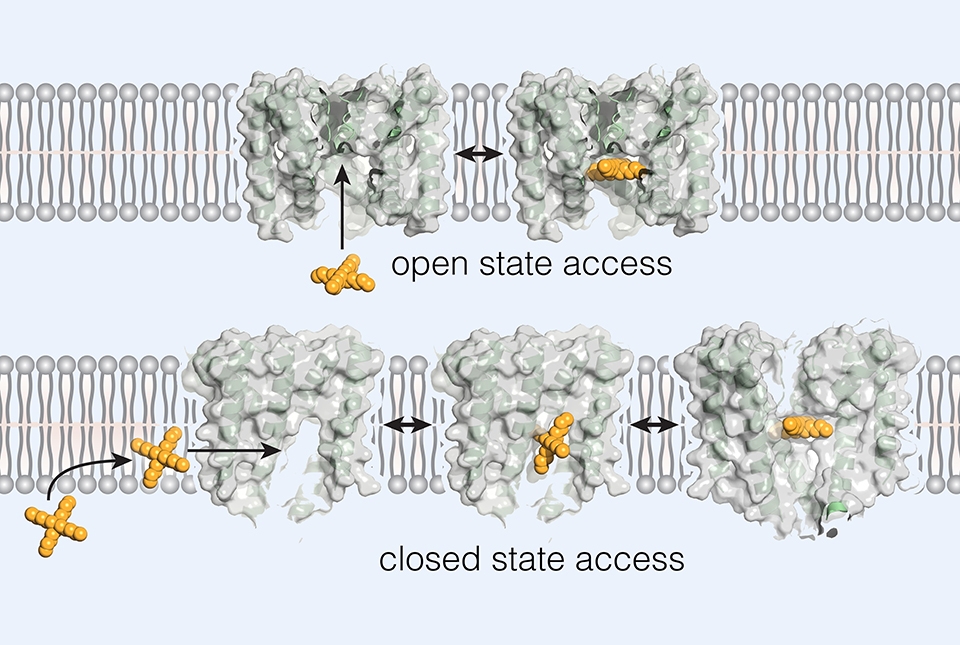
Dr. Nimigean and her team have been exploring the structure and function of BK channels, both directly and with experiments on a bacterial version called MthK that is easier to study in the laboratory. Recently, they observed that a family of MthK- and BK-blocking compounds—not suitable as drugs but useful as laboratory tools—can access and effectively plug the MthK channel, or “pore,” even when structural imaging shows that the entrance to the pore is fully closed.
“Since these compounds wouldn’t have direct access to the pore in this closed state, we wondered how they were able to get in,” Dr. Nimigean said.
To resolve this conundrum, the researchers turned to structural imaging methods, experiments with normal and mutated MthK, and, in Dr. Allen’s laboratory, computer modeling of the interactions between the channel-blocking compounds and the MthK ion channel.
They discovered that when MthK is in the closed state, the structure develops large openings on its sides through which the MthK-blocking compounds can access the ion-conducting pore. These openings are inside the cell membrane, so the MthK-blocking compounds must first travel a short distance into the membrane to reach them.
The researchers also observed from existing structural data that side-openings or “fenestrations” like those in the MthK channels are present in BK channels as well.
Scientists believe that BK-blocking or -activating drugs could help treat disorders such as epilepsy and hypertension. However, no selective BK channel-modulating drug exists, in part because little is known about how changes in the BK channel structure relate to the channel’s function. Another problem is drugs that affect BK channels also interact with other ion channels because they typically target the entrance to the potassium-conducting chute or “pore,” which is not very different from the pores of other types of ion channels. Such indiscriminate interactions could lead to havoc in the body.
“These fenestrations are unique to BK-type channels, which suggests that future drugs targeting these sites could work as selective BK channel blockers or activators,” Dr. Nimigean said.
She and her team are planning follow up experiments in BK channels and hope to use their findings to discover selective BK channel-modulating compounds that could be developed into drugs.