The heart damage seen in many severely ill COVID-19 patients results in part from infection-activated immune cells called macrophages, which infiltrate the heart and secrete cell-damaging chemicals, according to a study co-led by researchers at Weill Cornell Medicine, NewYork-Presbyterian, Icahn School of Medicine at Mount Sinai and Columbia University Irving Medical Center. The research identifies new potential treatments for COVID-19 patients as well as describes a model system for further studies and drug screening.
In the study, published April 15 in Circulation Research, the researchers used a laboratory system to grow different kinds of cells together to study the heart-damaging process in COVID-19. They were also able to use the system to identify two existing United States Food and Drug Administration (FDA)-approved drugs that may be able to prevent or ameliorate this type of cardiac complication in COVID-19 patients.
“There have been prior studies of COVID-19 pathology using animal models, but an advantage of our model is that it can use human-derived cells and can be adapted to screen for potential therapies,” said co-senior author Dr. Shuibing Chen, Kilts Family Associate Professor of Surgery at Weill Cornell Medicine.
The research, which began at the outset of the COVID-19 pandemic, was a collaboration involving several other co-senior authors: Dr. Todd Evans, associate dean for research and Peter I. Pressman, M.D. Professor in Surgery at Weill Cornell Medicine; Dr. Robert Schwartz, associate professor of medicine in the Division of Gastroenterology and Hepatology at Weill Cornell Medicine; and virologists Dr. Benjamin tenOever of the Icahn School of Medicine at Mount Sinai and Dr. David Ho of Columbia University Irving Medical Center.
Although COVID-19 is best known for causing pneumonia and respiratory distress, other organs are commonly affected, including the heart, which is damaged in an estimated 20 to 30 percent of patients hospitalized with COVID-19, and is associated with worse outcomes for such patients. The cause of this damage hasn’t been clear.
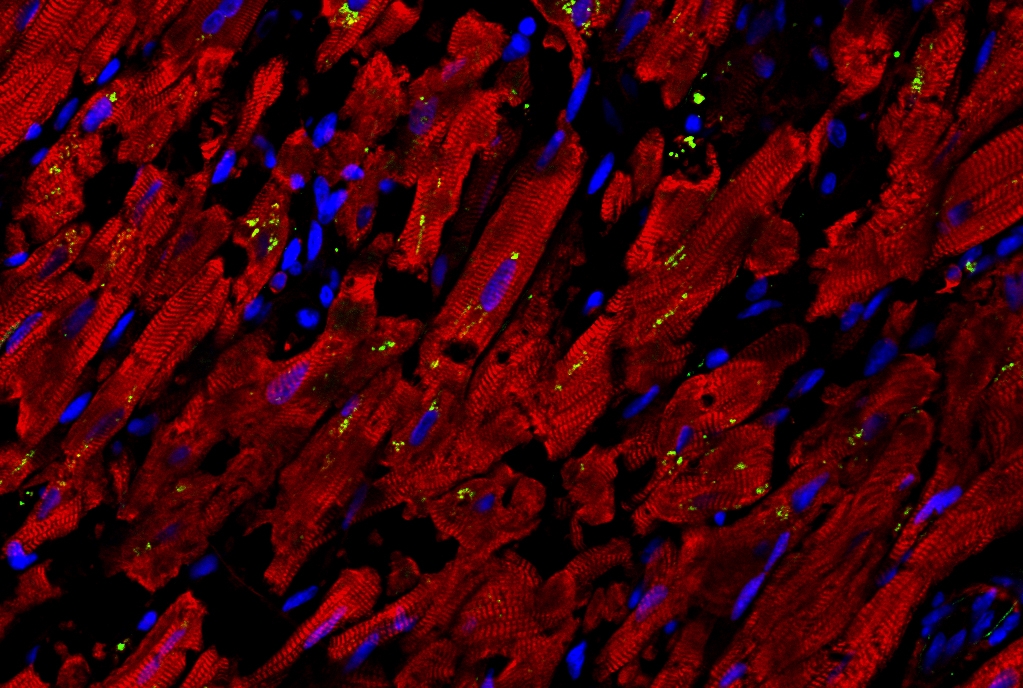
For the study, Dr. Schwartz and his colleagues compared the autopsied hearts of 15 patients who had died with COVID-19 and 11 who had died of unrelated causes. They found that the heart muscle cells from the COVID-19 patients had signs of strikingly high activity of inflammatory genes, including the gene for CCL2, an immune signaling protein that has been implicated in many inflammatory conditions and is known to summon macrophages.
“Consistent with the findings on gene expression, we observed a significant infiltration of macrophages into the heart tissue in the COVID-19 cases,” Dr. Schwartz said.
The finding was also consistent with those from animal studies, which have suggested that SARS-CoV-2, the coronavirus that causes COVID-19, can infect heart cells and trigger the release of CCL2. But the researchers sought a model system that would better model human physiology and be amenable to automated methods for quickly testing thousands of potential treatments.
Dr. Chen and Dr. Evans therefore pooled their expertise in cell-based disease models, and engineered a lab-dish culture of human stem cell-derived cardiac cells and macrophages. They found that adding the SARS-CoV-2 virus led to infection of the cardiac cells, secretion of CCL2 from the infected cells, and a resulting increase in macrophage activity. The macrophages themselves were not infected by the virus, but in their inflammatory state they released highly reactive and damaging oxygen molecules that triggered the death of many of the cardiac cells.
“Based on our previously published data, we believe cardiac cells are probably also directly injured and killed in COVID-19 by infection with the SARS-CoV-2 virus, and recruitment of macrophages when they infiltrate the heart is probably doing some good by removing infected and dying cells,” Dr. Evans said. “However, this new study suggests that when macrophages go into this hyper-inflammatory mode, the damage they cause can account for a large part of the pathology seen in autopsied hearts from COVID-19 patients.”
The researchers lastly used their COVID-19 model to search for potential treatments. They tested a collection of nearly 1,300 drugs that are already approved for other uses by the FDA and found two that robustly reduce the levels of reactive oxygen molecules and reduce the deaths of cardiac muscle cells.
One of the drugs, ranolazine, is a drug used to treat chronic chest pain (angina) from narrowed coronary arteries. The researchers found evidence that ranolazine reduces the activity of macrophage genes that are responsible for producing reactive oxygen molecules.
The other drug, tofacitinib, normally is used to treat rheumatoid arthritis and other serious inflammatory conditions. The researchers found that it protects cardiac cells in their model essentially in the same way it works against inflammatory conditions: by inhibiting a signaling pathway called the Janus kinase pathway, which is required in many aspects of immune activation.
A similar Janus kinase-inhibiting drug, baricitinib, has been shown in a clinical trial to shorten recovery times for hospitalized COVID-19 patients, and last November was approved by the FDA for emergency use in such cases.
The researchers hope that the potential treatments they identified can be tested in clinical trials. They plan to continue, in the meantime, to develop cell-culture models for the heart and other organ systems affected in COVID-19.
Dr. Robert E. Schwartz is a paid scientific advisory board member for Miromatrix Inc. and paid consultant and speaker for Alnylam pharmaceuticals, Inc.