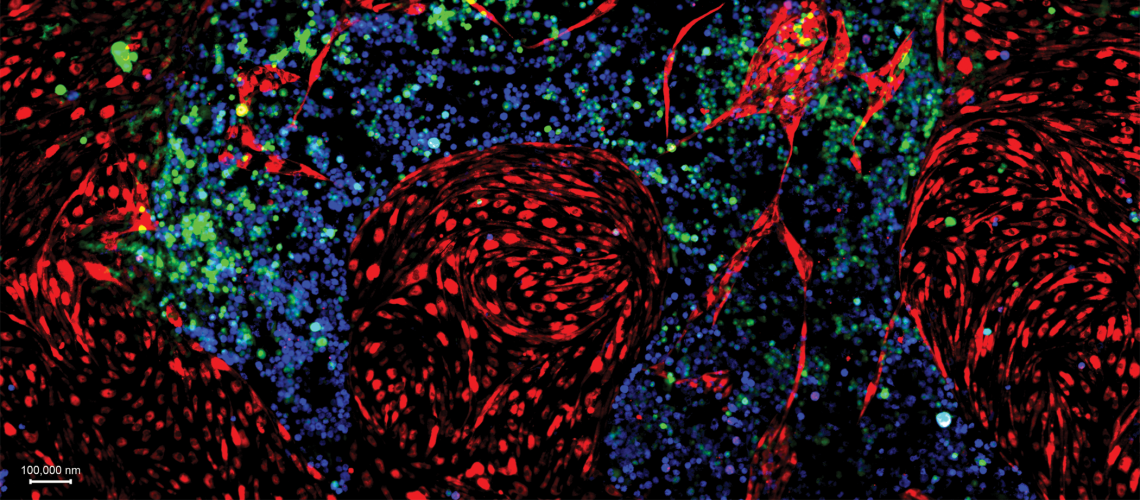
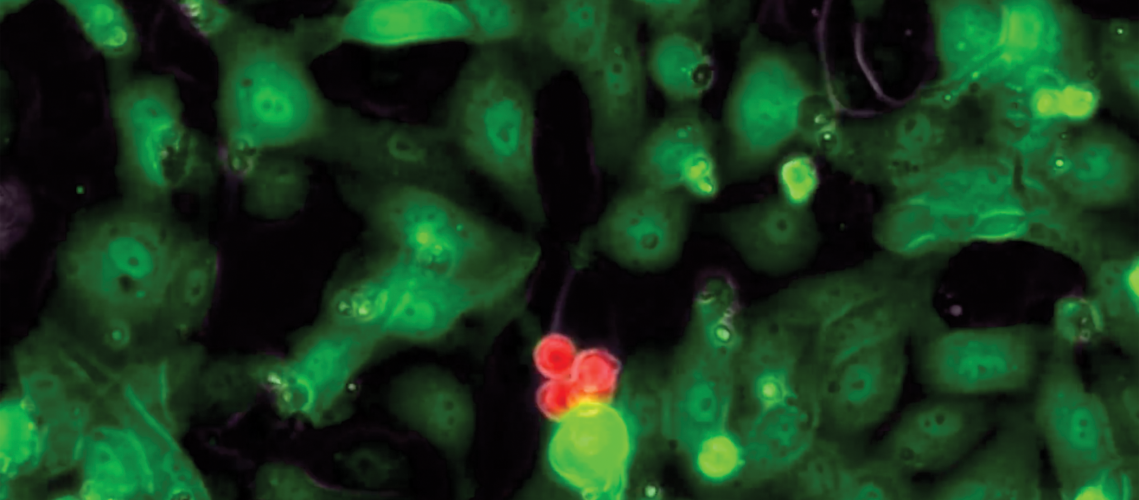
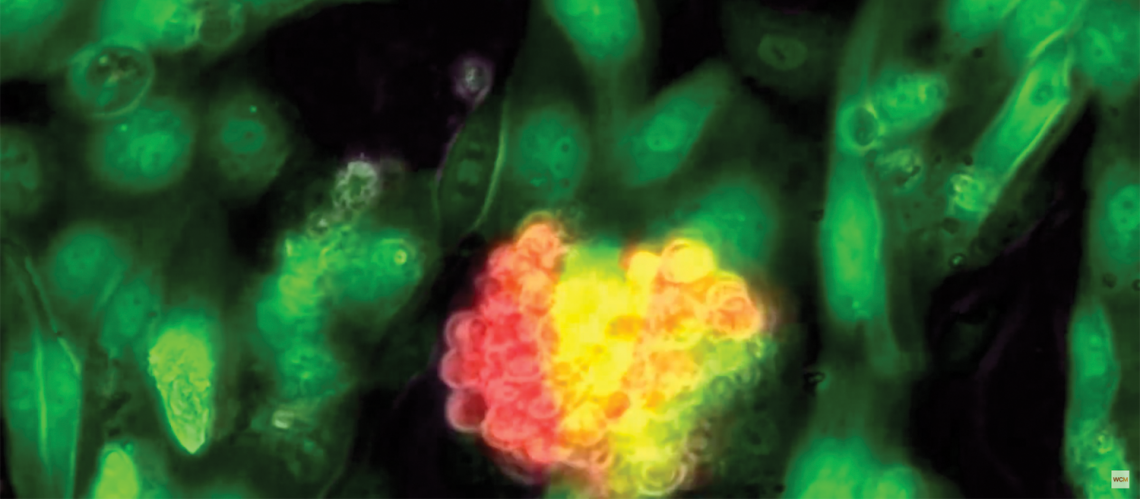
The proof they were seeking was close; the researchers knew that much. After years of trying—years in which the lights on the west side of the Ansary Stem Cell Institute and Division of Regenerative Medicine deep inside Weill Cornell Medicine made a small and unyielding dot on the predawn Manhattan skyline—their experiment had unfolded as Dr. Shahin Rafii and his team had long theorized. Inside a small Petri dish yards away from Dr. Rafii’s office, human blood stem cells capable of forming into any type of blood cell had multiplied time and again in a carefully curated environment. Viewed through a powerful microscope, the cells seemed to dance and glow as they grew in number, four becoming eight, then 16, then 32. On it went.
It was, in short, a breakthrough. The cells’ reproduction would go on to be the subject of two articles in Nature and to advance scientific thought on how blood diseases might someday be treated. But seeing something of this magnitude with their own eyes, as they did on an afternoon in July 2014, was not enough: the task of a pioneering researcher is not simply to trigger a phenomenon, but to then explain precisely how and why it occurred. They were, after all, on the path to assert that long-lasting blood stem cells could be grown outside of a human body. Even as their own doubts dissipated, they knew that questions would soon come from all sides.
Dr. Rafii’s lab, like so many cutting-edge research centers across the globe, is a landscape of hedged bets and fleeting euphoria. There are no bed-bound patients or pacing relatives begging for cures. Its warren of rooms sit largely quiet. Refrigerators hum; fluorescent lights glow; the hour of day is elusive. This, its greatest drama—cells bopping about on a monitor, an excited cluster of people pointing and talking—is rather muted, considering the potential clinical implications.
But imagine these landmark blood cells grown in Dr. Rafii’s lab bound for a cancer-stricken child—her bone marrow ravaged, her blood bereft of the T-cells central to life itself, her body wan and unable to help itself grow strong again, hope dimming. Globally, more than 300,000 children receive a cancer diagnosis each year and 80,000 die of the disease, according to the American Childhood Cancer Organization. And “a large number of patients who could be cured by a bone marrow transplant do not have a suitable donor,” says Dr. Joseph Scandura, scientific director of the Myeloproliferative Neoplasms Center, part of the Division of Hematology and Medical Oncology at Weill Cornell Medicine, an oncologist at NewYork-Presbyterian/Weill Cornell Medical Center, and a senior co-investigator in the project.
Here on Dr. Rafii’s screen was a possible new weapon against the carnage of cancer. The idea: extract a healthy cell from the patient themselves, multiply it in the lab, then send those cells back into the body to facilitate healing. “If we can do this,” Dr. Zev Rosenwaks, chief of reproductive medicine at Weill Cornell Medicine, recalls saying to Dr. Rafii, “then we have an unlimited source of stem cells for curing the patient. We can turn a single stem cell that is destined to become a blood vessel into blood.” Cracking the code of how a blood stem cell is triggered to repopulate, the scientists believed, could ultimately lead to a simple, bold statement that eludes many dedicated scientists like Dr. Rafii over an entire lifetime of research: this could someday form a new cure.
Colleagues underscore how much is at stake. Dr. Michel Sadelain, director of the Center for Cell Engineering at Memorial Sloan Kettering Cancer Center and an associate professor of immunology in medicine and in pediatrics at Weill Cornell Medicine, agrees. “The pursuit of hematopoietic stem cell expansion is one of the grand goals of biomedical research today,” he says. “If we were able to generate large numbers of unadulterated blood-forming stem cells, a number of diseases—from cancer to autoimmunity and more—could be better treated.”
Dr. Nancy Speck, an investigator at the Abramson Family Cancer Research Institute at the University of Pennsylvania’s Perelman School of Medicine, notes the all-or-nothing risk inherent in Dr. Rafii’s quest. “Shahin is trying to develop new technology—that could have potential clinical application,” she says. “It’s a risky proposition and requires a lot of courage to go down that path. However, the payoff could be enormous.”
Various researchers in this story have relationships with Angiocrine Bioscience that are independent of Weill Cornell Medicine.
This story first appeared in Weill Cornell Medicine, Vol. 17. No. 1