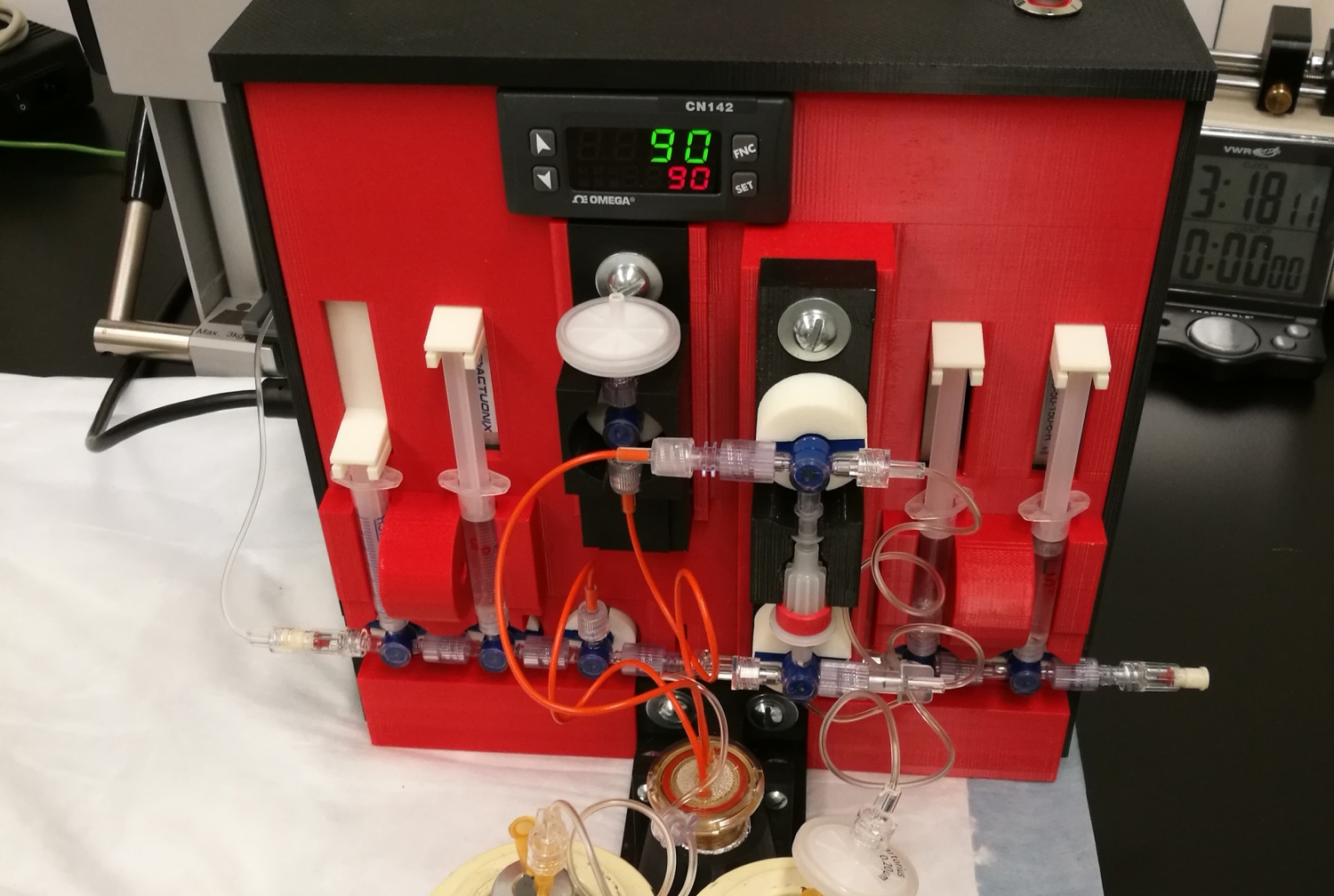
Weill Cornell Medicine scientists have built the first 3-D-printed automated synthesis units (ASUs) for producing customized radioactive drugs.
Made from a combination of 3-D-printed, electronic and robotic parts, the prototype ASUs are smaller and significantly cheaper to manufacture than commercially available machines. The cutting-edge innovation may help address the increasing demand for radiopharmaceuticals used to detect and image cancer and cardiac conditions.
In a paper published Sept. 19 in Science Advances, Dr. John Babich, professor of radiopharmaceutical sciences in radiology in the Division of Radiopharmaceutical Sciences and Molecular Imaging Innovations Institute (MI3), Department of Radiology at Weill Cornell Medicine and colleagues describe how they designed and built four ASUs for making different radiopharmaceuticals.

Dr. John Babich
“There are several commercial machines available, but they are expensive, costing up to $250,000, and often require cumbersome modifications for custom drug manufacturing,” Dr. Babich said. “The cost of building our customized 3D-printed ASUs ranged from $1,200 to $7,000 each. We estimate that we can build 20 of our ASUs for the same cost as a commercial unit.”
Nuclear medicine involves injecting a patient with a small amount of a radioactive drug that adheres to or enters target cells and reveals what is happening inside the tissues through imaging. For example, a radioactive version of glucose is taken up by cancer cells, making them visible with positron emission tomography (PET) imaging. The radioactivity dissipates within minutes to hours, limiting toxicity to patients.
Since the radioactive material for making radiopharmaceuticals has a short half-life (i.e., the time it takes for half the material to disintegrate), it is necessary to start with a very large amount to end up with the small amount used for imaging a patient. The chemical reactions required to manufacture radiopharmaceuticals must take placed within a lead-lined protective hood called a “hot cell” to protect operators from radiation exposure during the manufacturing process.
The new 3-D-printed ASUs are each about the size of a toaster, much smaller than commercial machines, which are the size of a typical microwave oven. “The smaller size means that we can put several ASUs in a single hot cell and produce different drugs at the same time,” said Dr. Babich, who is also a member of the Sandra and Edward Meyer Cancer Center and the Citigroup Biomedical Imaging Center at Weill Cornell Medicine. “We could potentially manufacture a multitude of radiopharmaceuticals for our large clinical research program at Weill Cornell Medicine.”
Dr. Babich and colleagues used 3-D-printed parts made from plastic, such as acrylonitrile butadiene styrene or nylon, and porcelain. They combined the 3D-printed parts with electronic and robotic parts, depending on the processes they needed for making different drugs. As a result, the purpose-built ASUs are portable and weigh significantly less than commercial units that are made mostly of metal. The units were built with operator safety in mind with remote on/off switches that are controlled outside the hot cell using software run on a tablet computer.
The first and simplest ASU makes carbon-11 labeled fatty acids, radioactive tracers used to observe fatty acid metabolism in the heart and cancers. Next, the scientists engineered three more ASUs with increasing complexities. The second ASU was designed to carry out labelling of small molecules with fluorine-18. Here they focused on a target for prostate cancer imaging called prostate-specific membrane antigen (PMSA). Fluorine-18 may be a better alternative for meeting the increasing demand for prostate cancer imaging than the radioactive drug gallium-68, since it has a longer half-life and multiple doses can be made in a single production run. Fluorine-18 labeled compounds are currently under clinical evaluation for various conditions at numerous centers around the world.
The third ASU can be used to make various gallium-68 labeled compounds in considerably less time than commercially available units, resulting in less risk of operator error and improved production yields. For the fourth ASU, the scientists improved on the third ASU by eliminating the incubation step and incorporating a continuous flow approach, reducing the total reaction time for making gallium-68 from 15 minutes to five.
The researchers, including co-authors Dr. Alejandro Amor-Coarasa, now at the Albert Einstein College of Medicine, and Dr. James Kelly in the Division of Radiopharmaceutical Sciences and Molecular Imaging Innovations Institute (MI3), Department of Radiology at Weill Cornell Medicine and intend to produce radiopharmaceuticals and file investigational new drug applications with the U.S. Food and Drug Administration for their approval for use in clinical research.
“In addition to imaging applications, there is a growing interest in the medical oncology community for using radiopharmaceuticals for cancer treatment as alternatives to chemotherapy,” Dr. Babich said. “More efficient, customized, automated production becomes even more critical as therapeutic uses require significantly higher doses of radioactivity than is used for imaging purposes.”
The study was funded in part by the Clinical and Translational Science Center at Weill Cornell Medicine through a Cooperative Agreement awarded by the National Center for Advancing Translational Sciences, Grant No. 5 UL1 TR000457–07.
Dr. John Babich is the founder, with an equity stock interest, of Noria Therapeutics Inc., a radiotherapy company developing targeted therapeutic and imaging radiopharmaceuticals (alpha-emitting) for use in oncology. Dr. Babich is the inventor of various patents that have been licensed to Noria. Also, Dr. Babich has an equity stock interest in Ground Fluor Pharmaceuticals, Inc., a biomedical company developing proprietary new chemistry for the synthesis of pharmaceutical compounds for Positron Emitting Tomography (PET) imaging.