Treatment for rheumatoid arthritis (RA) has come a long way in recent years. In many cases, a battery of medications can now successfully stymy the inflammatory cells that cause swelling and pain when they infiltrate tissues around the joints.
Yet for some reason, about 20% of patients with painful, visibly swollen joints consistently get no relief from multiple rounds of even the strongest of these anti-inflammatory drugs.
Surgical interventions intended to remove inflamed tissue have revealed why: “In some cases, their joints aren’t actually inflamed,” said senior author Dr. Dana Orange, an associate professor of clinical investigation in Rockefeller’s Laboratory of Molecular Neuro-oncology. “With these patients, if you press on the joint, it feels mushy and thick to the touch, but it’s not caused by the infiltrating immune cells. They have excessive tissue growth, but without inflammation. So why are they experiencing pain?”
In a new paper, published April 10 in Science Translational Advances, she and her colleagues suggest an explanation. These patients have a suite of 815 genes that activate abnormal growth of sensory neurons in tissues that cushion the affected joints.
“These 815 genes are rewiring the sensory nerves, which explains why anti-inflammatory drugs don’t work to alleviate pain for these patients,” Dr. Orange said. The findings may lead to new treatments for these outliers.
A puzzling disconnection
Rheumatoid arthritis is a tricky chronic disease. Its symptoms—stiffness, tenderness, swelling, limited motion, and pain—slowly emerge in the hands, wrists, feet, and other joints. It occurs symmetrically (not just in one hand but in both, for instance) and sporadically, with irregular flare-ups. Extreme fatigue and depression are also common.
Most cases of RA are caused by products of immune cells such as cytokines, bradykinins, or prostanoids invading the synovium—a soft tissue lining the joints—where they bind to damage-sensing pain receptors. Drugs that target immune mediators have made RA a far more tolerable condition for most, but those suffering from the disconnection between inflammation and ache haven’t benefitted.
Doctors often prescribe these patients drug after anti-inflammatory drug in an ultimately fruitless attempt to give relief. As a result, “we are subjecting some patients to a lot of medications that cause immunosuppression and yet have little chance of making their symptoms better,” Dr. Orange said.
She and her colleagues sought answers in the genes expressed in the joint tissue samples of these patients.
Genetic culprits
The researchers looked at tissue samples and self-reported pain reports from 39 patients with RA who had pain but little inflammation. They also developed a machine-learning analysis that they coined graph-based gene expression module identification (GbGMI).
GbGMI tests every possible combination of genes in a dataset to determine the optimal number of genes that together associate with a targeted clinical feature—in this case, pain.
Using RNA sequencing, the researchers found that of the 15,000 genes expressed in the tissue samples, about 2,200 had increased expression in the 39 patients. Using GbGMI, they identified 815 genes that together associated with patient report of pain.
“This is a challenging problem as we have a large number of genes but a limited number of patients,” said co-senior author Dr. Fei Wang, professor of population health sciences and founding director of the Institute of Artificial Intelligence for Digital Health at Weill Cornell Medicine. “The graph-based approach we used effectively explored the collective associations between a gene set and patient-reported pain in this case.”
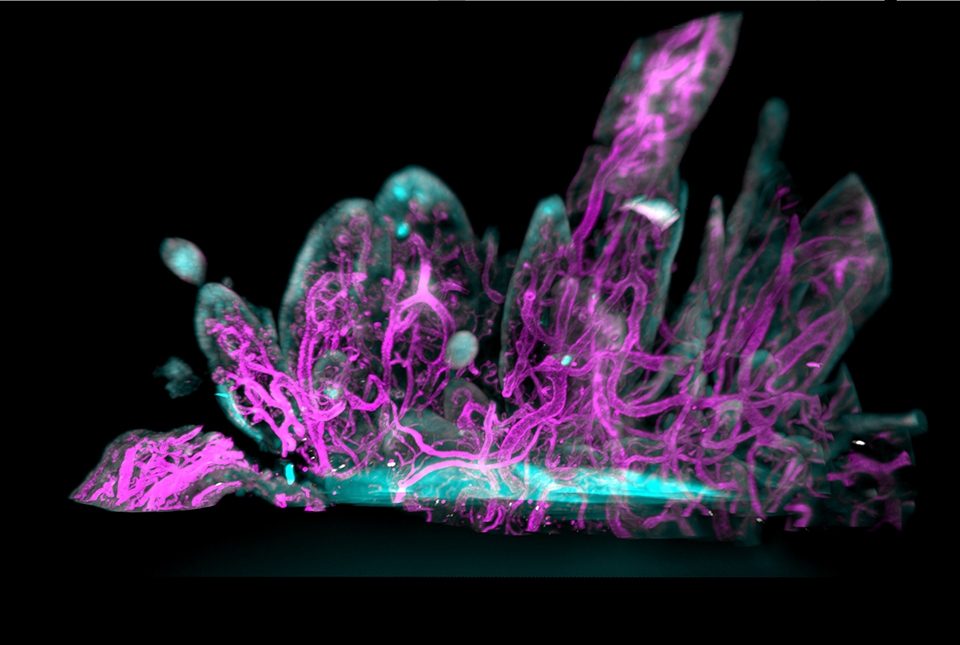
Single cell sequencing analysis found that of the four types of fibroblasts in synovial tissue, CD55+ fibroblasts exhibited the highest expression of pain-associated genes. Located in the outer synovial lining, CD55+ cells secrete synovial fluid, allowing for frictionless joint movement. They also expressed the NTN4 gene, which codes for a protein called Netrin-4. Proteins in the netrin family guide axon growth paths and promote new vascular growth.
Surprising pain pathways
These genes, it turned out, were enriched in pathways that are important for neuron axon growth, the researchers discovered. The keys to sensation, sensory neurons receive and transmit information to the central nervous system. Axons are the tendrils that branch out from them into tissues.
“That led us to hypothesize that perhaps the fibroblasts are producing things that alter the growth of sensory nerves,” she said.
But what role was the protein playing in the sensation of pain?
To find out, they grew neurons in vitro and then doused them with Netrin-4, which sparked the sprouting and branching of CGRP+ (gene-related peptide) pain receptors. It’s the first time that Netrin-4 has been shown to alter the growth of pain-sensitive neurons, Dr. Orange noted.
Imaging of RA synovial tissue also revealed an overabundance of blood vessels, which feed and nurture new cells. These vessels were encased by CGRP+ sensory nerve fibers and were growing towards the lining fibroblasts in areas of excessive tissue growth, or hyperplasia. This process likely leads to the squishy swelling that many rheumatologists and surgeons have mistaken for inflammation.
Better drugs
In the future, the researchers aim to home in on other products that fibroblasts may be producing that can affect the growth of pain-sensitive neurons. They’ll also delve into the other types of sensory nerves that might be affected.
“We studied one type, but there are about a dozen. We don’t know if all nerves are affected equally. And we don't want to block all sensation. Sensory nerves are important for knowing that you should avoid certain movements and the position of your joint in space, for instance,” Dr. Orange said.
“We want to drill down on those details so that hopefully we can come up with other treatments for patients who don't have a lot of inflammation. Right now, they’re taking medications that can cost $70,000 a year but have no chance of working. We must do a better job of getting the right drug to the right patient.”
This story originally appeared on the Rockefeller University website.
Many Weill Cornell Medicine physicians and scientists maintain relationships and collaborate with external organizations to foster scientific innovation and provide expert guidance. The institution makes these disclosures public to ensure transparency. For this information, please see the profile for Dr. Fei Wang.
The project discussed in this story was funded in part by National Science Foundation grant number 1750326; the National Institute of Arthritis and Musculoskeletal and Skin Diseases grant numbers R01AR078268, UC2AR081025, R01AR077019, R01AR064251, R01AR060364, P30AR079206, the National Institute of Neurological Disorders and Stroke grant number U19NS130608; The National Center for Advancing Translational Sciences grant number UL1TR001866; and the Accelerating Medicines Partnership Program: Rheumatoid Arthritis and Systemic Lupus Erythematosus (AMP RA/SLE) Network. Funding for AMP RA/SLE work was provided through grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (UH2AR067676, UH2AR067677, UH2AR067679, UH2AR067681, UH2AR067685, UH2AR067688, UH2AR067689, UH2AR067690, UH2AR067691, UH2AR067694, and UM2AR067678).