White blood cells called neutrophils have an unappreciated role in eradicating solid tumors, according to a surprise discovery from a team led by Weill Cornell Medicine scientists.
In the study, published March 30 in Cell, the researchers investigated how a T cell-based immunotherapy was able to destroy melanoma tumors even though many of the tumor cells lacked the markers or “antigens” targeted by the T cells. They found that the T cells, in attacking the tumors, activated a swarm of neutrophils—which in turn killed the tumor cells that the T cells couldn’t eliminate. The findings could lead to new immunotherapies that harness this unexpected but potent antitumor immune response.
“We have tended to think of innate cells as immune cells that, at best, can help stimulate an initial T cell response to a tumor. In addition, many studies have shown that neutrophils support tumor progression, but here we reveal that they can have a critical role in eliminating and finishing off a tumor that would otherwise escape a T cell therapy,” said study co-senior author Dr. Taha Merghoub, deputy director of the Sandra and Edward Meyer Cancer Center, the Margaret and Herman Sokol Professor of Oncology Research and a professor of pharmacology at Weill Cornell Medicine, and co-director of the Ludwig Collaborative Laboratory.

Dr. Taha Merghoub (left) and Dr. Jedd Wolchok (right). Credit: Travis Curry
“This work clearly shows us that the monolithic term ‘neutrophil’ needs to be more specific, based on the use of single-cell technology,” said co-senior author Dr. Jedd Wolchok, the Meyer Director of the Meyer Cancer Center and a professor of medicine at Weill Cornell Medicine, co-director of the Ludwig Collaborative Laboratory and an oncologist at NewYork-Presbyterian/Weill Cornell Medical Center. “In the past, this general term referred to a population of cells that were not thought to be helpful in controlling tumors. We now know that a subset of these cells is critical in optimizing engineered T cell therapies to overcome heterogeneity.”
Cancer immunotherapies, which harness or boost immune cells’ ability to attack malignant cells, have begun to revolutionize cancer treatment over the past decade. In some cases, these therapies have essentially cured advanced cancer patients who would have had no hope of survival otherwise. Yet for most cancers, immunotherapies are effective in only a minority of patients. In general, researchers still have much to learn about how anticancer immunotherapies work and how their effectiveness can be improved.
In the study, the researchers investigated an experimental immunotherapy that includes a drug to boost T cell activity and proliferation, plus T cells that have been engineered to recognize a melanoma-associated antigen. Tumors sometimes can evade an immunotherapy targeting a specific tumor antigen simply by ceasing to express that antigen—the tumor cells that don’t express the antigen are called “escape variants.” However, the researchers found that their boosted T cell therapy could eliminate melanomas, in standard mouse models, even when a large portion of the melanoma cells lacked the targeted antigen, Trp1.
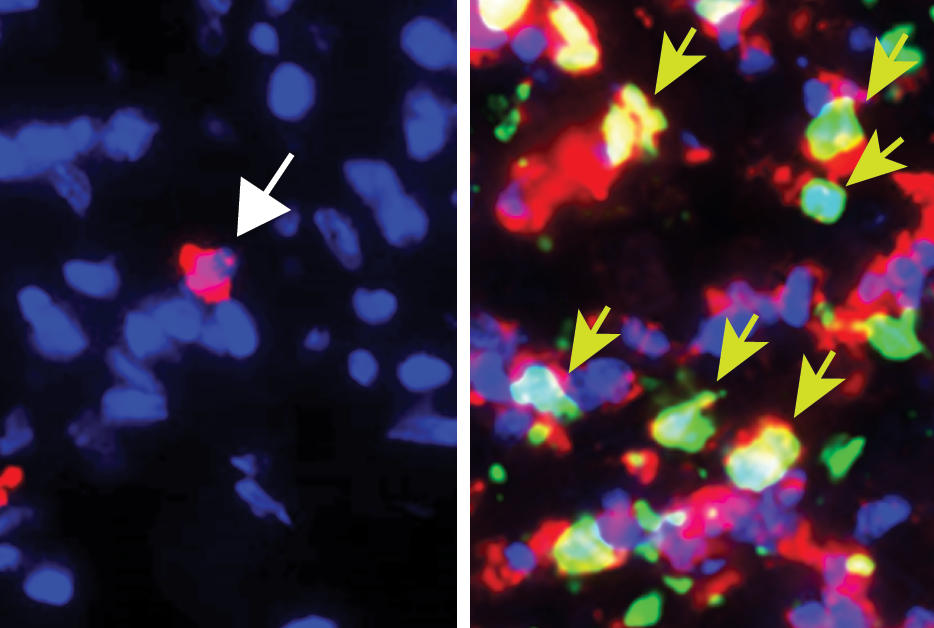
Ultimately, they determined that the initial anti-tumor activity of the T cells against Trp1-expressing melanoma cells triggered a secondary tumor-killing response—from neutrophils. These white blood cells are best known as first-responders to infections and wounds. As members of the evolutionary older “innate” immune system, they do not target specific antigens in the way that T cells do. Yet the researchers concluded that the neutrophils summoned by their T-cell immunotherapy were indeed responsible for killing off the remaining, non-Trp1-expressing melanoma cells—at least in part by secreting the highly reactive molecule nitric oxide.
As part of the study, the researchers identified a characteristic gene expression pattern in the antitumor neutrophils, and found that in a widely used database on melanoma patients, the greater presence of this gene-expression “signature” in biopsied tumor samples was associated with better outcomes for patients.
The results were especially surprising because prior studies have shown that neutrophils around a tumor often act as allies of the tumor—the tumor co-opts them to help it survive and spread, which they do in part by suppressing other elements of antitumor immunity.
In any case, the new findings suggest that in the context of a strong immunotherapy that includes engineered T-cells targeting tumor antigens, and a general boosting of T-cell functions, neutrophils can play a significant antitumor role—in fact, an essential role in mopping up escape variant tumor cells that would otherwise keep the tumor alive.

Dr. Daniel Hirschhorn
“Conventional T cell-based therapies have been successful in treating cancers, but they are not as effective against heterogenous tumors, which have antigen escape variants that can be invisible to the immune system,” said Dr. Daniel Hirschhorn, an assistant professor of research in pharmacology at Weill Cornell Medicine. “It was incredibly surprising to discover that T cell-educated neutrophils can eliminate these ‘invisible’ tumor cells. This discovery highlights the importance of mobilizing multiple arms of the immune system in the fight against cancer.”
The researchers now are continuing to study these anti-tumor neutrophils, to determine how they can best be induced and directed—as cancer fighters on their own, or as enhancers of other immunotherapies.
“I also hope that we can find a way to use measures of these antitumor neutrophils in tumor biopsies as biomarkers that help us choose the best therapies for patients,” Dr. Merghoub said.
Many Weill Cornell Medicine physicians and scientists maintain relationships and collaborate with external organizations to foster scientific innovation and provide expert guidance. The institution makes these disclosures public to ensure transparency. For this information, see profile for Dr. Wolchok.