By Amy Crawford
Portraits by John Abbott
When Bob Azopardi arrived for his first appointment at the Sandra and Edward Meyer Cancer Center at Weill Cornell Medicine and NewYork-Presbyterian Hospital in October 2010, he was desperately ill. It had been 10 years and many rounds of chemotherapy since he was first diagnosed with chronic lymphocytic leukemia, a type of non-Hodgkin's lymphoma. His oncologist had told him that he'd done all he could. "My doctor had gone through his whole bag of tricks," recalls Azopardi, who'd been forced to give up his X-ray repair business as his health declined. "I was told to put my house in order, because this was it."
With swollen lymph nodes the size of oranges in his neck, under his arms and pressing on his sciatic nerve, Azopardi was in constant pain and could walk only with difficulty. At the age of 60, he was preparing to say goodbye to his wife of two years — high school sweethearts, they'd rekindled a romance at their 35th reunion — when a colleague of his oncologist suggested he see Dr. Richard Furman, director of Weill Cornell Medicine's Chronic Lymphocytic Leukemia Research Center. "It took three people to get me on the table," Azopardi recalls. "Dr. Furman looked at me and said, 'You're a very sick man, but I do have a clinical trial, and if it works for you, you'll be dancing in six weeks.' I said, 'Dr. Furman, have you ever thought about seeking psychiatric care?' He said, 'Trust me.' "
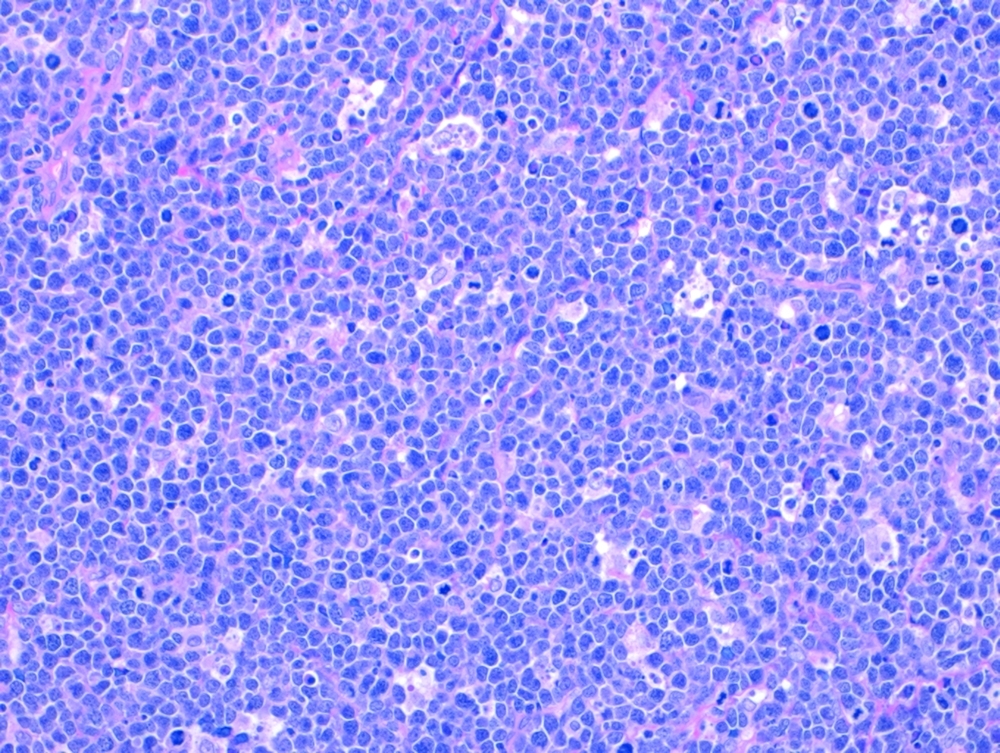
Burkitt lymphoma: Tumor cells under the microscope.
It turned out that Dr. Furman's promise was nearly correct: a little more than four weeks after starting on a new, once-daily pill called ibrutinib, Azopardi walked unassisted for the first time in nearly two years. The 2010 trial he participated in was just one of dozens that have taken place at Weill Cornell Medicine's Center for Lymphoma and Myeloma over the past two decades. Still thriving more than four years later, Azopardi is just one of countless patients whose lives have been saved.
Physicians diagnosed about 80,000 new cases of lymphoma in the United States in 2014, attributing 20,000 deaths to the disease. On the upside, five-year survival rates have been rising steadily. In the more common non-Hodgkin varieties, for example, survivability has risen from less than 50 percent in 1990 to more than 70 percent in 2011. That's the last year for which statistics are available, but lymphoma specialists say that rates have improved even more in just the past few years, thanks in large part to drugs like ibrutinib, which saved Azopardi's life. Now marketed under the trade name Imbruvica, it was approved by the FDA in 2013. "There have been several major breakthroughs, and what excites me is that we're actually turning them into new treatments for patients," says Dr. John Leonard, associate dean for clinical research, the Richard T. Silver Distinguished Professor of Hematology and Medical Oncology, and chair of the Lymphoma Committee of the National Cancer Institute's Alliance for Clinical Trials in Oncology, which helps set the national agenda for cancer research. Plus, he notes, because lymphoma is principally treatable with drugs — not surgery — research on the disease often leads to discoveries that spur progress in the fight against other cancers.
Dr. Leonard — along with Dr. Furman and Dr. Morton Coleman, a clinical professor of medicine and the center's former director — was among the first Weill Cornell Medicine physicians to get involved in trials of lymphoma drugs, back in 1997. Since then, Weill Cornell Medicine has helped to develop a series of breakthrough treatments, including nearly every lymphoma drug that the FDA has approved in the past 15 years. It's a track record that Dr. Leonard chalks up to the institution's ability to attract the best doctors and researchers in the field, and encourage them to work together to tackle what was historically one of the toughest problems in oncology. Among the core group of veterans, Dr. Leonard says, are Dr. Coleman (who remains an active member of the voluntary faculty) and Dr. Furman, who has been at Weill Cornell Medicine for 20 years. "There have been some key clinical faculty who have been here awhile, and we've trained or recruited some additional ones," he says. "We've got a critical mass of people who are involved, and I think it feeds on itself. You get more opportunities, people know you, they approach you, you build collaborations. This has led to partnerships with a number of talented laboratory researchers, and even greater progress."
Lymphoma's Long History
Lymphoma is cancer of the lymphatic tissue, part of the body's immune system that includes the lymph nodes, spleen, thymus gland, and bone marrow. It originates from abnormal lymphocytes, a type of immune cell, and it can spread throughout the body or remain, in so-called indolent form, in the lymphatic system. It was known in the early 19th century; in 1823, the English physician Thomas Hodgkin described the class of lymphomas that would bear his name. By the 1960s, lymphoma was routinely treated with chemotherapy and radiation, but these therapies could have devastating side effects that in some cases were worse than the disease. Beginning in the '90s, medicine began to make serious headway, improving understanding of the myriad types of lymphomas and how they differ, and then developing therapies precisely targeted to each disease.
First came monoclonal antibodies, artificial versions of immune system proteins that bind chemically to surface markers on specific types of cells. The technology, in development since the '70s, had helped refine the classification of lymphomas during the '80s and early '90s, leading to its therapeutic use to tag cancer cells and recruit the body's own immune system to eradicate tumors. In 1997, when the FDA approved a monoclonal antibody called rituximab for B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia, the new drug offered the first proof that immunotherapy could be an effective cancer treatment. "That was the thing that heralded the move beyond standard chemotherapy," says Dr. Peter Martin, the Charles, Lillian, and Betty Neuwirth Clinical Scholar in Oncology and director of the Clinical Research Program in Lymphoma. "Oncology was entering a new era."
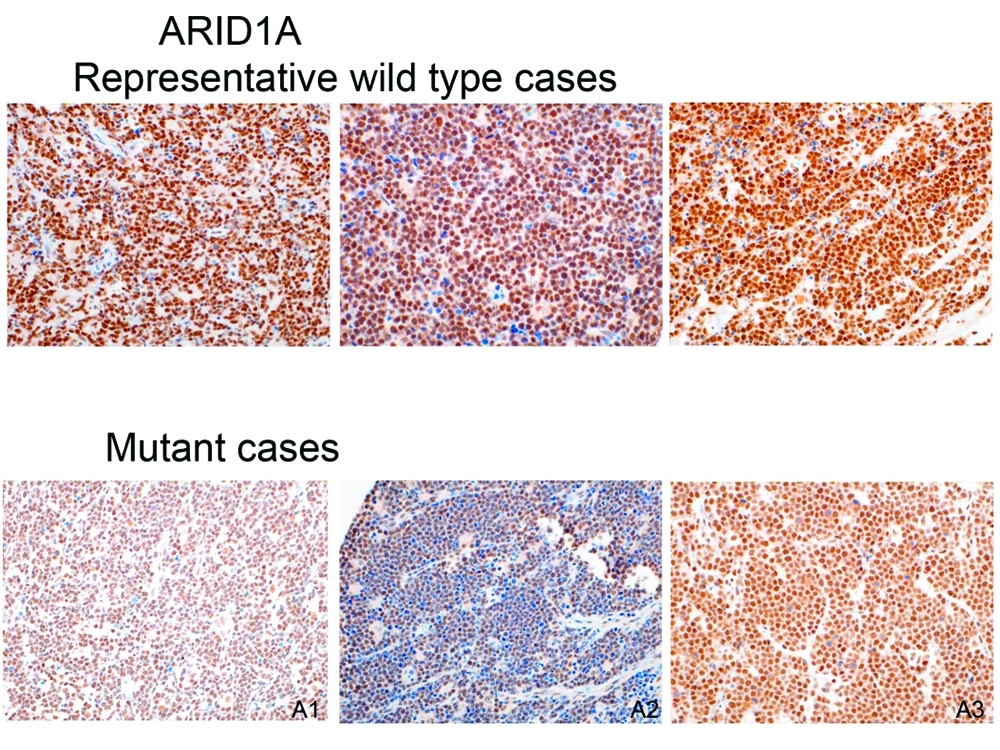
Protein staining demonstrating that some Burkitt tumors lack the ARID1A protein, which may help explain why they're more aggressive and resistant to chemotherapy.
Monoclonal antibodies have been followed by other classes of targeted therapies, including immunomodulatory drugs (IMiDs) like lenalidomide and thalidomide — although the latter causes birth defects in pregnant women, it's an effective treatment for certain cancers — and small-molecule kinase inhibitors, which block chemical signaling to interfere with lymphoma cells' ability to survive and grow. Because they are so precisely targeted, these new drugs have far fewer side effects than either chemo or radiation, which kill healthy cells along with cancerous ones.
One kinase inhibitor, ibrutinib, is the drug Dr. Furman used to treat Azopardi. It blocks an enzyme called Bruton's tyrosine kinase, which is necessary for the maturation of B-cells, part of the immune system that becomes cancerous in most non-Hodgkin lymphomas. In 2013, the results of clinical trials at Weill Cornell Medicine and elsewhere — including the one for which Azopardi volunteered — showed that patients with mantle cell lymphoma and chronic lymphocytic leukemia, both incurable, responded remarkably well to ibrutinib. The FDA labeled it a "breakthrough drug" and accelerated its approval for patients who had had at least one prior therapy. But even as the lymphoma community celebrated, a team of Weill Cornell Medicine researchers led by Dr. Furman made a troubling discovery: lymphoma cells could eventually mutate and become resistant to the drug. Those findings led them to embark on new clinical trials to look at a second-generation version of ibrutinib and to study how it performs in combination with other drugs. Drs. Leonard, Coleman, Furman and Martin have been intimately involved in the development of some of the most promising molecules of the past decade — ibrutinib included — as have colleagues Dr. Lisa Roth, an assistant professor of pediatrics, and Dr. Jia Ruan, an associate professor of clinical medicine who received her doctorate from Weill Cornell Medicine in 1998 and her medical degree in 1999.
Dr. Furman adds that it appears patients are less likely to become resistant when they have not previously been exposed to chemotherapy, which means that the standard of care for certain lymphomas might one day leave out chemo entirely — an exciting prospect not only because it has devastating side effects but also because the cancers quickly develop resistance to it. "Patients die from their disease becoming refractory to treatment, or due to the toxicities of the treatments themselves," explains Dr. Furman, the Morton Coleman, MD, Distinguished Associate Professor of Medicine, adding that life expectancy was once 10 years after the start of treatment. "We still need long-term data, but these new agents even work in chemotherapy refractory disease. The ability to work when chemotherapy does not, and without toxicities, indicates the tremendous potential that exists for these new agents. I have patients who came to me at death's door five years ago who are alive and well and have wonderful quality of life. Now, I can honestly say I hardly ever lose a patient. It's certainly very nice for an oncologist to be in that position."
Rewriting Faulty Instructions
As good news continues to come from the front lines, researchers at the Meyer Cancer Center and elsewhere at Weill Cornell Medicine are also at work in the lab, trying to improve understanding of the disease and searching for even safer and more effective treatments. Dr. Roth, head of the newly created Adolescent and Young Adult Lymphoma Program, notes that lymphoma is the most common cancer among people between the ages of 18 and 30. While current therapies are often very effective for this population, young adults also have different needs — preserving their fertility, for example — so the potential for better treatments is exciting. "In the laboratory, we're looking at a large panel of drugs that haven't made their way to clinical trials yet," says Dr. Roth, the Charles, Lillian, and Betty Neuwirth Clinical Scholar in Pediatric Oncology. "What is most radical and new is how we screen these drugs."
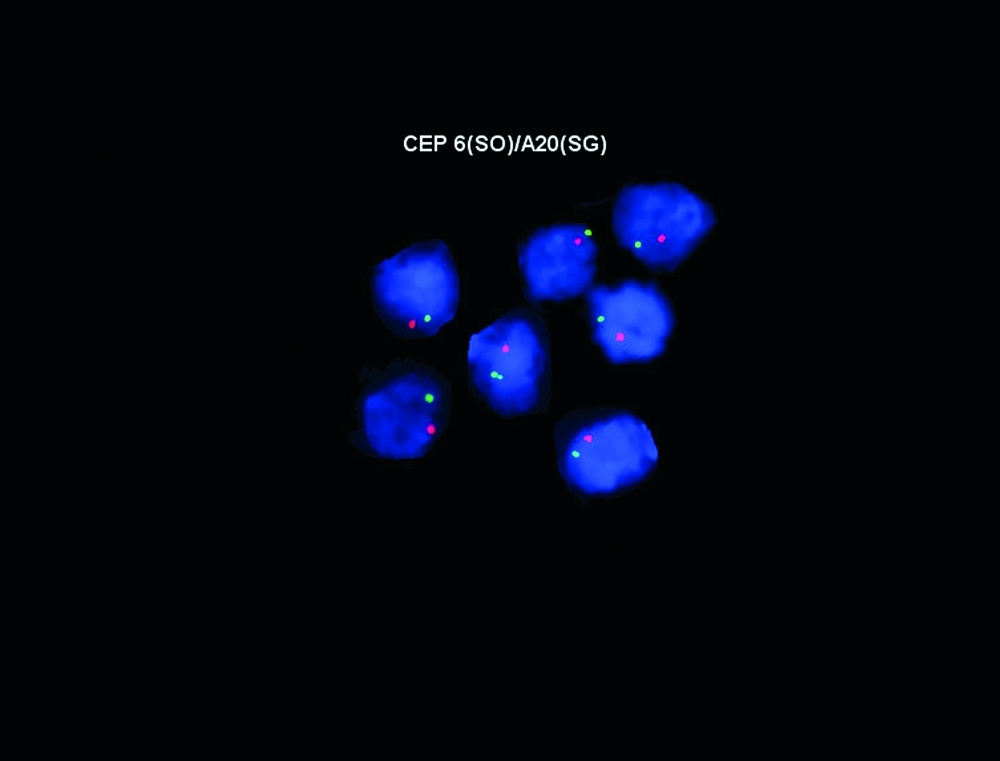
All aglow: Fluorescent probes staining a newly described gene, A20, important in Burkitt lymphoma.
In the past, lymphoma has been modeled in the lab using mice that have been injected with lines of human lymphoma cells. These cell lines were derived from patients as far back as the '50s and maintained in the lab, but because of their age they no longer replicate in the same way that lymphoma grows in people. "That's why a lot of these drugs make it to clinical trial and fail," Dr. Roth explains. But Weill Cornell Medicine researchers now maintain a bank of lymphoma samples from recent patients, which can be injected directly into mice. "We have these systems up and running now to mimic, in a much more accurate manner, what would likely happen in a patient," Dr. Roth says. "We can take a patient's tumor and put it into a mouse and then evaluate these new drugs to see which one is most effective."
The most promising direction for drug development may lie with the epigenome, the set of chemical switches that modify how cells read their genetic codes. "You can think of the genome of DNA as being like the computer hardware," explains Dr. Ari Melnick, the Gebroe Professor of Hematology-Oncology and director of the Raymond and Beverly Sackler Center for Biomedical and Physical Sciences. "Computer hardware is kind of inert on its own, but epigenetics is the cell software, the instructions that control how cells behave."
Sometimes, this software contains errors that cause cells to proliferate out of control: they become cancerous. That's especially likely to happen in B-cells, Dr. Melnick says. "B-cells are our defense mechanism — they can't fool around," he says. "So in order for normal B-cells to make antibodies to the bugs that infect us, they undergo radical and rapid changes. That's all controlled epigenetically." But sometimes, instead of helping B-cells adapt to fight new pathogens, these changes switch on instructions that tell cells to proliferate or that keep them from dying when they're no longer needed. Such errors may mean the patient develops lymphoma. But drugs could rewrite these faculty instructions.
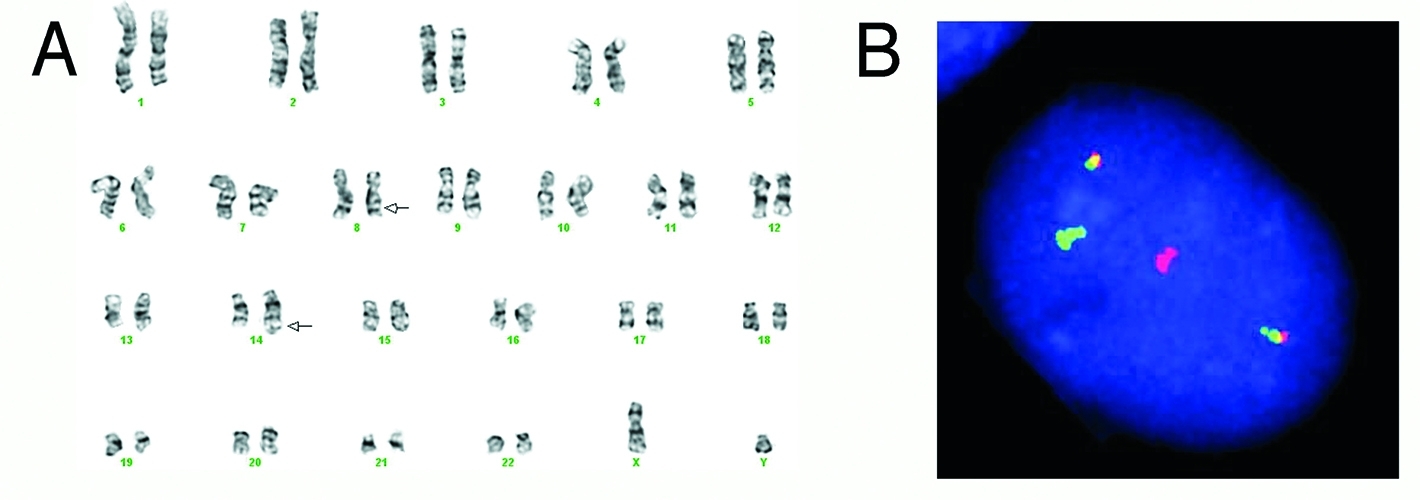
Chromosomes of a Burkitt tumor (A) and fluorescent probes (B) showing its classic chromosomal change.
Epigenetics are coordinated by regulatory proteins — including, in B-cells, a molecule called BCL6. Normally, when the immune system is fighting a pathogen, this protein coordinates key sets of genes to increase B-cell production. However, growing B-cells have a tendency to develop mutations that maintain BCL6; the end result is that B-cells grow out of control and transform into lymphomas. Dr. Melnick and his team are designing drugs that inhibit BCL6 without harming the immune system, and they hope to have a new therapy ready for clinical trials within a year or two. "All those instructions that are required for lymphoma disappear when we target BCL6 with our inhibitors," Dr. Melnick says. "Without BCL6, the lymphoma cells can't survive; they just dissolve."
Other Weill Cornell Medicine and Meyer Cancer Center labs are working different epigenetic angles. Dr. Leandro Cerchietti, the Raymond and Beverly Sackler Research Scholar and an assistant professor of medicine, has spent many years developing a new protocol for treating diffuse large B-cell lymphoma, a common but highly aggressive subtype. In chemotherapy-resistant forms of the disease, epigenetic alterations switch off genes that normally trigger cell death, effectively silencing them by adding chemicals called methyl groups to the DNA. But Dr. Cerchietti and colleagues discovered that a drug called azacitidine could remove these methyl groups and switch the genes back on, making subsequent chemo more effective. It took five years to figure out how to make this work in humans, but eventually they settled on a five-day regimen that led to complete remission in 11 out of 12 patients in a 2013 proof-of-concept trial.
The drug, Dr. Cerchietti explains, reprograms the lymphoma into a less aggressive disease — a technique that could prove effective in many different cancers. "Over the past years there has been an increasing understanding of the molecular basis of lymphomas that fueled an explosion of therapeutic opportunities," Dr. Cerchietti says. "The biggest challenge we face nowadays is how to rationally translate them to cure more patients." To that end, Dr. Cerchietti and his colleagues are developing strategies against lymphomas based on characteristics that extend to the molecular level. In addition to testing these new targeted therapies in lab mice, they are working with the College of Veterinary Medicine on the Ithaca campus, looking at how new drugs might help fight spontaneous lymphomas in pet dogs; investigators include research oncologist Dr. Kristy Richards, who has a joint appointment at both institutions. "This is an incredibly challenging endeavor, but it is also extremely rewarding," Dr. Cerchietti says, "and it's made possible by a highly motivated and collaborative team of scientists and physicians."

Dr. John Leonard (left) and Dr. Leandro Cerchietti
Although more breakthroughs may be on the horizon, lymphoma remains a challenging disease. For Dr. Martin, the story of a patient he treated several years ago sums up both the excitement and the frustration of working in the field today. His patient, an older man with mantle cell lymphoma, had come to Weill Cornell Medicine to participate in a clinical trial of ibrutinib after his disease had failed to respond to the standard treatments. "He was starting to become quite sick, losing a lot of weight," recalls Dr. Martin. "He had a lot of lymphoma in his lungs, and his breathing was getting worse quickly. The day that he was due to start the clinical trial, he had to be hospitalized."
A few doses into his treatment, the patient choked on a pill because tumors were compressing his airway and affecting his ability to swallow. He stopped breathing, and after doctors resuscitated him he wound up intubated in the ICU. "It was a real challenge, because the lymphoma was the reason he was so sick — and the only means for treating it was with the ibrutinib, but there was no way we could give him an oral therapy while he was intubated," Dr. Martin remembers. "We spent a day really not knowing what was going to happen. Then in a moment of delirium the patient pulled the tube out, and, surprisingly, his breathing was okay. He was able to swallow the pills and, in a very short period of time, improved dramatically, so that six months later the lymphoma was mostly gone. Nine months later he was hiking in the mountains. This was a man who had literally almost died in the hospital — unbelievable."
Two years later, the patient's lymphoma returned, and this time he succumbed. "That story really sticks with me for a couple of reasons," Dr. Martin explains. "One, because of all of the work that people did to develop this drug, this guy had two years that would have been taken away from him. You have this incredible celebration of life, a victory." Dr. Martin pauses, smiles, and shakes his head. "But then, on the other hand, there's still so much work left to do."
A version of this story first appeared in Weill Cornell Medicine, Vol. 14, No.2.

