Researchers at Weill Cornell Medicine have gleaned new insights on the ways cells maintain the tips of their chromosomes, or telomeres. The findings help illuminate a wide range of phenomena, from cancer development to fungal evolution.
The study, published Dec. 16 in Communications Biology, could offer new targets for cancer therapy.
All organisms with linear chromosomes consisting of two intertwined strands of DNA, including humans, must confront the problem of replicating and protecting their chromosomes' telomeres. For the cell, the challenge is that a telomere end looks a lot like one of the free ends of a broken chromosome.
"When the cell sees a telomere as a double-strand break, it will try to repair it, and the way it repairs these breaks is by fusing them to other pieces of DNA," said senior author Dr. Neal Lue, professor of microbiology and immunology and a member of the Sandra and Edward Meyer Cancer Center at Weill Cornell Medicine.
Fusing telomeres to other chromosomes can cause drastic difficulties in chromosome partitioning during cell division, sending the cell down the pathway toward cancer development. Indeed, many cancers show exactly these types of chromosome fusions.
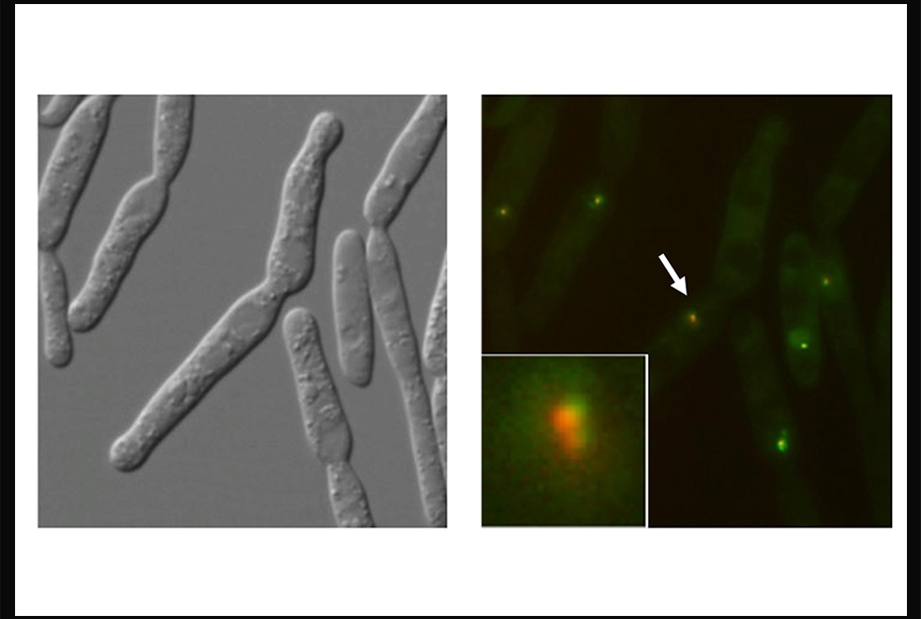
To prevent that, and also to ensure that the telomeres replicate properly, cells employ a variety of specialized telomere-binding proteins. Dr. Lue and his colleagues study these proteins in the corn smut fungus Ustilago maydis. "This particular fungus has a DNA repair system that is close to the mammalian system," said Dr. Lue, adding that it includes several close relatives of important human DNA repair and telomere-binding proteins. However, U. maydis is much easier to manipulate in the lab than human cells, making it an ideal model organism.
Dr. Lue's team, with collaborators from the lab of Dr. William Holloman, also professor of microbiology and immunology and a member of the Meyer Cancer Center at Weill Cornell Medicine, focused on the activity of two telomere proteins, Tay1 and Trf2, which help replicate and protect telomeres using different molecular pathways. Disrupting the activities of either protein caused the cells to develop distinct telomere replication or maintenance errors as they divided. Tay1 works by controlling the function of BLM helicase, a DNA repair protein that can separate the intertwined DNA strands at telomeres to enhance replication or recombination.
The results provide strong evidence that defects in these telomere proteins can drive abnormal DNA strand synthesis, separation and splicing. Dr. Lue says his lab is now investigating the possibility of targeting analogous mechanisms in mammalian cells, with an eye toward suppressing the telomere maintenance capacity of cancer cells.
The work also highlights a striking example of convergent evolution, in which a critical mechanism gets reinvented multiple times in different evolutionary lineages. Human cells, for example, appear to use another telomere protein rather than Tay1 to control the critical function of BLM helicase. Indeed, there is growing evidence that telomeres are extraordinarily malleable and adaptable in evolution. Thus, brewer's yeast, Saccharomyces cerevisiae, and its cousins have evolved entirely different telomere sequences since diverging from the fungal lineage that includes U. maydis, while mammalian cells have maintained the same telomere sequence as U. maydis but replaced some of the associated proteins.
"Initially the fungi had a telomere sequence that was the same as mammalian telomeres, but later on in some branches, the telomere sequence deviates, and accompanying this you have the remodeling of telomere proteins, said Dr. Lue. “The cells somehow manage to evolve alternative telomere binding proteins to carry out these important functions."