For three decades, Dr. Stephen Hoffman has pursued a single goal: a malaria vaccine
By Beth Saulnier
While studying malaria at the Naval Medical Research Institute in the early '90s, Dr. Stephen Hoffman '75, offered himself up as a test subject. As a means of understanding the mechanisms of host defense, Dr. Hoffman rolled up his sleeve, inserted his arm into a containment box, and let himself be bitten by thousands of mosquitos carrying Plasmodium falciparum, the parasite that causes the disease.
But these weren't just any insects: they'd been irradiated to weaken the P. falciparum sporozoite, the form of the parasite that infects the host. A few months later, he and 14 fellow volunteers again let themselves be bitten — this time, by mosquitos carrying the parasite in its virulent form. Thirteen of those hardy souls — Dr. Hoffman included — never got sick.
The idea that a weakened (or "attenuated") version of P. falciparum could confer immunity lasting months or years wasn't new; that understanding dated back to the '70s. But though Dr. Hoffman and his colleagues were hard at work trying to develop a malaria vaccine, they didn't consider the attenuated sporozoite concept a contender. After all, the sporozoites would have to be painstakingly harvested from the insects' miniscule salivary glands — in quantities sufficient to produce a vaccine, and without transmitting other potential pathogens to humans. Other avenues seemed much more promising.
Fast forward to the early Aughts: After two decades in the vaccine trenches, Dr. Hoffman had retired from the military and was working for Celera Genomics, the biotech firm famous for sequencing the human genome. Reviewing the reams of data from his Navy days, he was drawn back to those irradiated mosquitos. At a scientific meeting, he floated the idea. "I asked people in the room, 'What do you think about making this a vaccine?' and you could hear a pin drop," he recalls. "Nobody thought it was possible.'
Dr. Hoffman has spent the past decade and a half proving them wrong. Now, his Maryland-based biotech firm Sanaria, is in the midst of clinical trials on an attenuated sporozoite vaccine that has shown strikingly high efficacy rates of as much as 100 percent. "Steve is totally driven by the goal of developing a malaria vaccine. You can see it in his eyes; he is the most focused person you will ever want to meet," says Dr. Anthony Fauci '66, director of the National Institute of Allergy and Infectious Diseases — the NIH agency that has long been one of Sanaria's funders. "He has defied conventional wisdom multiple times and has always turned out to be correct. Whether he is going to get to the goal line, I do not know — but I think he is, actually. Knowing him, his incredible perseverance and innate capabilities, I think he will get there. If anybody is going to do it, Steve is."
Sanaria has held seven trials so far, with a dozen more — in the United States and Europe as well as in such African countries as Mali, Tanzania, Kenya, Ghana and Burkina Faso — imminent or under way. In June, the company announced that a series of trials in Equatorial Guinea, comprising thousands of subjects and running through 2018, would be funded by $48.5 million from that nation's government and three American oil companies. "It has been an extraordinary journey," Dr. Hoffman says. "We were told when we started that it was impossible to make this vaccine; we've made it. We were told that it was going to be impossible to administer it; we've shown that it's easy. We were told that it would be impossible to get it out to the people who need it most; we're showing that these challenges can be overcome."
A Moving Target
Unlike some infectious diseases — say, measles — surviving malaria doesn't confer lifetime immunity. However, each successive infection manifests less severely. Adults who grew up in malarial regions generally don't get sick enough to go to the hospital — but infants and small children suffering their first few bouts are exquisitely vulnerable. According to the World Health Organization, the disease took the lives of more than 400,000 people in 2015, the majority of them under five years of age. "In the last 50 years or so, malaria has been limited to tropical areas. And within the past decade, combined efforts — insecticide-treated bed nets, socioeconomic development, and new drugs — have driven it down by around half," notes Dr. Kirk Deitsch, a professor of microbiology and immunology at Weill Cornell Medicine who has studied the disease for decades. "But that's still hundreds of thousands of kids dying every year."

World traveler: Before he cut off his ponytail and joined the Navy, Dr. Hoffman (far right) and classmate Dr. Roger Hicks (far left), studied disease in Colombia
And malaria is an ever-moving target. P. falciparum is devilishly challenging: It has a multi-stage life cycle that involves not only the mosquito but the human liver and bloodstream. As each new generation of drugs beats it back, the parasite evolves to dodge it. "What we have lacked, historically, has been a vaccine to avoid the problem of drug resistance," Dr. Deitsch says. "That's where Steve's work comes in — to circumvent this continuous, up-and-down relationship that the human population has with malaria."
Dr. Hoffman's commitment to battling malaria traces, in part, to a faculty member who influenced so many Weill Cornell Medicine students of his era: Dr. Ben Kean, the legendary professor of tropical medicine. Inspired by Dr. Kean's mentorship, as well as that of distinguished public health professor Dr. Walsh McDermott, Dr. Hoffman and a classmate spent a summer in Colombia, studying diarrhea in malnourished infants. That experience prompted the pair to take a year off to travel around South America — to gain an understanding not only of the developing world, but of themselves. "Today, that's not unusual," he notes. "In 1972, it was unheard of."
Dr. Hoffman came down with amoebic dysentery three times; in Ecuador, he spent 10 days in the hospital with typhoid fever — refusing the first medication offered to him because its adverse side effects had been drilled into him in pharmacology class. "His focus and commitment very early in his career were remarkable," says Dr. Warren Johnson, the B.H. Kean Professor of Tropical Medicine, one of Dr. Hoffman's faculty advisers. After completing a family practice residency at UC San Diego — a choice Dr. Hoffman made because he believed the discipline would have the broadest application abroad — he heard about a group of doctors in Indonesia doing compelling research on infectious diseases such as typhoid fever, cholera and malaria. But there was a catch: They were in the Navy. "I had a beard and a ponytail and had been protesting the Vietnam War," he recalls. "I didn't have it in my mind to join the United States military. But in the spring of 1980, I cut off my ponytail, took the oath of office and went directly to Jakarta."
There, he and his colleagues did groundbreaking research — including a double-blind, placebo-controlled trial on identifying and treating patients at high risk of dying from typhoid. In 1984, the New England Journal of Medicine published the results, documenting a drop in mortality from 55 percent to 10 percent. Brimming with confidence, he developed a similar approach for treating malaria — but the death rates didn't budge. Says Dr. Hoffman: "I had many tens, perhaps hundreds, of children with malaria die whom I was taking care of."
Those losses spurred him to return to the United States to focus on malaria research — and follow the path that ultimately led to the founding of Sanaria and the current vaccine trials. With the latest World Health Organization figures tallying 214 million new cases of malaria worldwide in 2015, the stakes are almost unthinkably high. "Whoever develops the first effective malaria vaccine will go to Stockholm for their prize," says Dr. Henry Murray, the Arthur R. Ashe Jr. Professor of Medicine, who invited Dr. Hoffman to campus for the 2014 B. H. Kean-Boxer Family Foundation Lecture in Global Health. "He is just as likely as anyone else to do this. He has been laboring in the vineyards for more than 20 years on this concept and has shown nothing but tenacity, creativity and professionalism. I have great admiration for him — it's just that, like for anyone else, it's a tough nut to crack."
Great Expectations
When Dr. Hoffman started tackling that challenge in the mid-'80s, he and his Navy colleagues mainly focused on developing a "subunit" vaccine, one that targets specific proteins on the sporozoites' surface. Expectations were heady. "In 1984," he says with a wry laugh, "we thought we were going to win the Nobel Prize in the next two years." In 1986 — the first of two times he volunteered as a test subject — he received the experimental subunit vaccine himself. A few months later, he was exposed to malaria and was so confident he'd be protected that he jetted off to a conference in San Diego. Shortly thereafter, he recalls, "I called my wife and told her I had the flu She said, 'No, you idiot; you have malaria.' I was in denial, because we thought we were going to solve malaria right then and there."
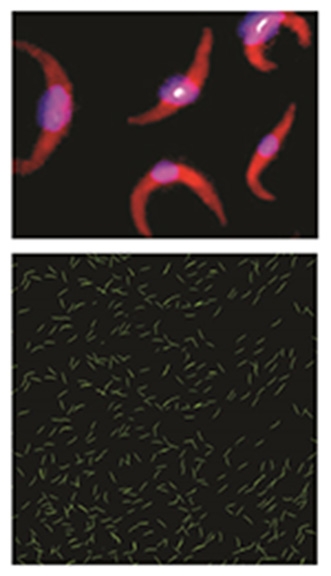
Invading force: Two views of the weakened Plasmodium falciparum sporozoite, the basis for Sanaria’s vaccine
After working on the vaccine effort from various angles for years, Dr. Hoffman left the Navy and went to Celera — though he never stopped thinking about malaria, and convinced the company to aid the fight by sequencing Anopheles, the genus of mosquito that transmits the disease. When he concluded that the attenuated sporozoite angle was promising — despite the fact that many in the scientific community considered it untenable — he founded Sanaria in summer 2003 with a $550,000 grant from the National Institutes for Health. Even the start-up's name cast it in opposition to the disease: Malaria translates as "bad air" in Italian, while Sanaria roughly means "healthy air."
The company's first home was an 800-square-foot storefront next to a carpet shop in a shopping complex outside of Washington that, Dr. Hoffman notes, "was later described in National Geographic as a 'dismal strip mall.' " The effort got a major boost four years later with $30.7 million in grants from the Bill & Melinda Gates Foundation that allowed it to build a manufacturing facility. But its initial clinical trial stumbled; the vaccine only protected 12.5 percent of subjects, a far cry from the 80 percent goal. By 2010 the Gates Foundation had dropped out and money was drying up. But Dr. Hoffman was convinced that the problem wasn't the vaccine; it was the delivery method. And indeed, studies at the NIH in nonhuman primates showed that when it was injected into a vein — rather under the skin or in the muscle — the vaccine worked.
Dr. Hoffman pitched his colleagues on one more clinical trial; it was approved by the U.S. Food and Drug Administration, and the National Institute of Allergy and Infectious Diseases funded and conducted it. In August 2013, Science published the results that Dr. Hoffman had longed for: Of the six volunteers who'd received five doses of the vaccine intravenously, every one was protected. Even in the group that got four doses, six out of nine didn't get sick. "This was big time; this was big news," Dr. Hoffman says. "However, it's one clinical trial, with a small number of subjects, and you have to reproduce it."
Tackling a 'Tall Order'
While Sanaria's concept has shown great promise, it still faces some logistical hurdles. Administering the vaccine intravenously — especially to the youngest patients, the target population given their mortality risk — takes training. And the vaccine must be kept extremely cold, even as it's transported to remote tropical regions. Dr. Hoffman says that both issues have proven manageable, and that the ongoing trials will bear that out. "He has a great idea, and he hasn't given up," says Dr. Linnie Golightly, an associate professor of medicine in microbiology and immunology. "He has followed where the science led him. When there are naysayers, he continues to move ahead. Even when other people don't have his vision, he brings them to the table." A decade ago, when Dr. Golightly sought to launch a study in Ghana on the causes of cerebral malaria — work that is ongoing — Dr. Hoffman offered contacts and advice. She has been known to laud him during parasitology lectures to her second- year students, even showing a slide of him in the Sanaria lab. "It's people like Steve that inspire us all," she says. "He's a fabulous scientist, a wonderful doctor, and a champion for finding ways to solve a complex, worldwide menace."
Sanaria isn't alone in pursuing a malaria vaccine. Among the others in development, one — a subunit vaccine developed by pharma giant GlaxoSmithKline and trade-named Mosquirix — is farther along in the pipeline, having gotten scientific (though not regulatory) approval in Europe last summer. But it has come under fire for its relatively low efficacy, having been found to reduce infection in African infants by only about a third, even after four doses — raising questions, Dr. Deitsch says, as to whether resources are better spent on proven preventives like bed nets. "The attenuated sporozoite vaccine that Steve's been working on is, in principle, much more effective," he says. "Even with the logistical challenges, it's much better than every other vaccine that anyone else is trying."
Sanaria currently aims — and even Dr. Hoffman admits this is "a tall order" — to have a version of its vaccine licensed by the FDA in mid-2018. That one would be geared toward travelers and military personnel, who don't require long-lasting protection. "The second goal, and the more important one," he says, "is to have a vaccine that could immunize entire populations, to halt transmission of the parasite and eliminate malaria." And if that comes to pass, how will it feel for Dr. Hoffman to realize his life's work? "It's hard to even fantasize about what that might mean — to be able to stand there with a vial of licensed vaccine," he says. "I can't begin to think about the numbness and elation, at the same time. If I go back to the day I decided that I wanted to be a doctor, never in my wildest dreams did I think I'd be pursuing something with that great an impact."
This story first appeared in Weill Cornell Medicine, Vol. 15, No.1.

