Scientists at Weill Cornell Medical College and Cornell University have gained new insights into the function of biological machines that scoop up glutamate, the most common neurotransmitter in the brain, from the nerve synapse. Their findings may offer investigators new strategies to develop more effective treatments for stroke, traumatic injury and neurodegenerative diseases.
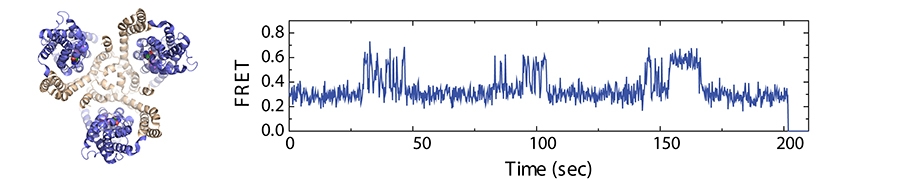
Static architecture of the glutamate transporter homologue, GltPh, (left) a trimeric protein comprised of a central scaffold (tan) and the three peripheral transport domains (blue). Real-time measurements of "elevator-like" motions of individual transport domains within GltPh as a function of time (right)obtained through single-molecule fluorescence imaging. Changes in fluorescence resonance energy transfer (FRET) efficiency reflect sub-nanometer (nm) to nanometer scale transport domain movements. Image credit: Drs. Scott Blanchard and Olga Boudker
“The brain — indeed life — depends upon the communication between neurons that is carried out by neurotransmitters,” said co-corresponding author Dr. Scott C. Blanchard, an associate professor of physiology and biophysics at Weill Cornell Medical College.
Communication requires the release of a small molecule, like the neurotransmitter glutamate, from one nerve cell, and its movement across a small gap (the synapse), to be recognized by another neuron. Then, the neurotransmitter must be cleared quickly from the synapse. The glutamate transporter clears glutamate from the nerve synapse, readying it for the next burst of glutamate that transmits a nerve impulse.
Surprisingly, this transporter is built much like an elevator, consisting of a scaffold embedded in the surface of specific cells in the brain, and a cabin that accepts the glutamate from the nerve synapse and delivers it back to the inside, where it can be recycled for subsequent bursts. When operating properly, this circulation of glutamate contributes to memory formation and cognition, but if it is unchecked, it can do much damage.
“Glutamate in the brain is essential for forming memories and for consciousness, but we know that if it isn’t recycled properly, the nerve cells can wither,” said Dr. Olga Boudker, co-corresponding author and an associate professor of physiology and biophysics at Weill Cornell. “Impaired transporter function is linked to neurodegeneration, brain damage following stroke and other disorders. We conducted this study to gain a deeper understanding of how the glutamate transporter actually performs its work.”
“To clear the neurotransmitter, the cabin has to cross the cell surface, over and over again, very rapidly,” Dr. Blanchard explained.
Using state-of-the-art technologies, the researchers were able to observe the movements of the pump in action, and their discovery is detailed in the Feb. 5 issue of Nature.
“This is a great first look at the mechanism,” added Dr. Jack Freed, the Frank and Robert Laughlin Professor of Physical Chemistry at Cornell University. “Our collaborative effort is the most detailed biophysical investigation of this machine’s actions that I am aware of.”
“The collaborative efforts that made these discoveries possible are special in that they involve highly specialized means of investigating physical properties of the transporter—including a way to reveal its architecture in terms of the position of the individual atoms from which it is built, and the ability to follow collective movements of the ‘elevator’ domains in space and time during function,” said Dr. Harel Weinstein, chairman of the Department Physiology and Biophysics and the Maxwell M. Upson Professor of Physiology and Biophysics at Weill Cornell, who participated in this study. “It is only by combining this kind of precise information and analyzing it with the most advanced computational methods that we can hope to understand how the transport process works, and translate this into guides for achieving improved drug therapies.”
“Indeed, what makes the results of this study so exciting is the promise for new therapeutics; a deeper understanding of how this transporter functions has provided key insights into how it might be regulated” Dr. Blanchard added. “Revealing the elevator mechanism the way we have and establishing a mechanistic framework for transport opens the door for exploring how therapeutic regulation of this transporter class can be achieved.”
Scott Blanchard, Roger Altman and Zhou Zhou are inventors of dyes used in the experiments described in this paper, and Cornell University has licensed intellectual property covering the dyes to Lumidyne Technologies, a company in which Blanchard and Altman hold equity.

