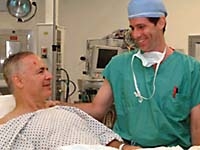
Gene therapy patient Nathan Klein (left) recovers after surgery with Dr. Michael Kaplitt.
History was made at NewYork Weill Cornell Medical Center on Monday, Aug. 18, when the world's first gene therapy for Parkinson's disease was performed on a 55-year-old New Yorker, Nathan Klein. Dr. Michael Kaplitt, assistant professor of neurological surgery at the Medical College and director of stereotactic and functional neurosurgery at NewYork-Presbyterian Hospital, performed the five-hour procedure, which also marked the first-ever in vivo gene therapy in the brain. The procedure for an adult neurological disease was part of a Phase I clinical trial approved by the Food and Drug Administration in October 2002.

Dr. Michael Kaplitt injects gene therapy virus into Nathan Klein.
The gene therapy procedure was announced at a press conference held at NewYork Weill Cornell on Aug. 20. Present were Dr. Antonio Gotto Jr., dean of the Medical College; Dr. Herbert Pardes, president and CEO of NewYork-Presbyterian Hospital; Dr. Philip Stieg, professor of neurological surgery and chairman of the Department of Neurological Surgery; Dr. Michael Kaplitt; Dr. Matthew During, professor of molecular medicine at the University of Auckland; Dr. Andrew Feigin, co-director of the Movement Disorders Center at North Shore University Hospital; and patient Nathan Klein and his wife, Claire Hamilton, and their 15-year-old twins.

Dr. Yu-Hung Kuo (left) watches as Dr. Michael Kaplitt (right) prepares for the infusion process.
Dr. Gotto, who gave the opening remarks, said, "Exciting things at an academic medical center are being exemplified today." Dr. Gotto emphasized that a cure for Parkinson's wasn't being announced, but "the first step in testing a new approach, which has not been tested before." Dr. Gotto also said: "This research represents a new approach to treating one of the most devastating diseases known to man."
"Nathan was awake for the entire operation, telling jokes that are not repeatable here," said a smiling Dr. Kaplitt. Mr. Klein was discharged from the Hospital prior to the press conference, and results from an MRI performed shortly before his release were "excellent," showing no fevers and no inflammation in the brain.
"Monday's surgery represents the realization of nearly 15 years of research in this area," said Dr. Kaplitt. "The goal of our gene therapy approach is to 're-set' a specific group of cells that have become overactive in an affected part of the brain, causing the impaired movements associated with Parkinson's disease. We hope that this trial, which is the first of its kind, will prove to be a safe treatment to allow gene therapy to move forward for Parkinson's disease and other brain disorders. In a month from now, if there are no adverse effects, we will proceed with the next patient," said Dr. Kaplitt.
With his family by his side, Mr. Klein acknowledged that the attention was overwhelming, but that he "felt great." To date, he has not felt any major differences from the procedure, and habitually suffers from a tremor on his right side ("my left side feels fine," he said); shuffling; trouble with balance; and a "soft voice," about which he said, "excuse me if I don't talk loud." Mr. Klein, a former TV producer, has been unable to work due to the debilitating disease.
In the procedure, Dr. Kaplitt pinpointed the optimal location in Mr. Klein's brain using information from an advanced 3T MRI image, which is subsequently merged with a CT scan, using the latest computer imaging technology. Then, the final target is confirmed using fine electrical probes that identify the signature pattern of electrical activity of individual cells within Mr. Klein's brain. With the target obtained, the gene therapy agent (adeno-associated virus or AAV) is slowly delivered through a very fine catheter. After a 90-minute infusion, the catheter is removed, the skin closed, and the patient sent to the recovery room.

From left: Dr. Andrew Feigin; Dr. Matthew During; Dr. Philip Stieg; Dr. Michael Kaplitt; patient Nathan Klein; Dr. Herbert Pardes; and Dr. Antonio Gotto.
AAV is the means by which the GAD (glutamic acid decarboxylase) gene enters the appropriate brain cells and begins production of a protein that produces GABA—a molecule that is released by nerve cells to inhibit, or dampen, activity. "Our intent, ultimately, is to normalize the chemical signaling of key affected brain areas in order to reduce the devastating effects of Parkinson's," said Dr. Kaplitt.
Dr. During, a co-investigator of the current clinical trial, along with Dr. Kaplitt, observes: "After years of focused effort, our technology and approach have reached a state of maturation where the risks are acceptable and the potential benefits sufficient to bring gene therapy to the clinic for neurological disorders. Nevertheless, we realize that this is but a first step in the long road of research and refinement toward developing effective gene therapies for the brain."

Dr. Antonio Gotto, dean of the Medical College.
As part of the Phase I trial, patients are being recruited and followed by Drs. David Eidelberg and Andrew Feigin at the North Shore University Hospital Movement Disorders Center. The initial trial will be limited to 12 patients with severe Parkinson's disease, of at least five-year's duration, for whom current therapies are no longer satisfactory.
Photos by Amelia Panico and Joan Penn.

