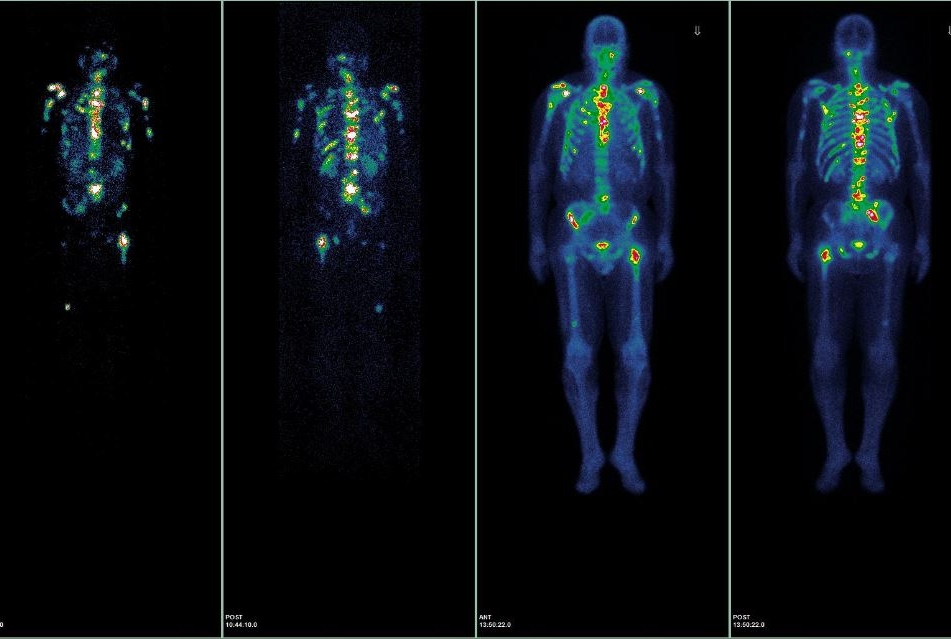
NEW YORK (May 31, 2017) — NewYork-Presbyterian and Weill Cornell Medicine have begun the first clinical trial in the United States that uses a small molecule to treat men with progressive prostate cancer that has spread beyond the prostate and is no longer responding to hormonal therapy. The Phase 1 study has completed its second round of patient enrollment, with the first six patients having undergone dosing. The researchers will be discussing the trial on June 5 at the 2017 American Society of Clinical Oncology meeting in Chicago.
The researchers are using the small molecule Lutetium 177Lu-PSMA-617 to target prostate-specific membrane antigen (PSMA), a protein that is abundantly expressed in 85-90 percent of metastasized prostate cancers. The small molecule binds to PSMA and delivers precise radiation therapy intended to shrink the cancer — even in cases in which cells have yet to form a visible tumor on a bone or CT scan.
The trial primarily seeks to determine the highest dose level of the drug that can be given without significant side effects. PSMA-targeted therapy is thought to be one of the most promising approaches in treating metastasized prostate cancer.
“This trial represents a new frontier in the treatment of metastatic prostate cancer,” said Dr. Scott Tagawa, medical director of the genitourinary oncology program at NewYork-Presbyterian/Weill Cornell Medical Center and The Richard A. Stratton Associate Professor in Hematology and Oncology at Weill Cornell Medicine. “While this type of therapy has shown promise, this is the first trial of its kind in the United States. So far, patients are doing well.”
While this trial is the first of its kind in the United States, this same approach to treat metastatic prostate cancer has gained traction in recent years in Germany, where physicians can treat patients who have exhausted standard treatment options under “Compassionate Use” laws. German physicians who are able to provide this treatment in a “Compassionate Use” setting have shown Lutetium 177Lu-PSMA-617 can reduce the volume of tumors in the body and lead to remission of the cancer.
NewYork-Presbyterian and Weill Cornell Medicine have been at the forefront of PSMA-targeted 177Lu therapy for more than a decade. Dr. Neil Bander, the Bernard and Josephine Chaus Professor of Urological Oncology at Weill Cornell Medicine and a urologic oncologist at NewYork-Presbyterian/Weill Cornell Medical Center, developed the first monoclonal antibodies that could bind to PSMA in prostate cancer cells. As a result of Dr. Bander’s efforts, PSMA has become recognized as the best known prostate-cancer specific cell surface molecular target. The lead antibody he developed, J591, was shown to be able to target virtually all prostate cancers in patients while also avoiding healthy tissue and normal organs.
NewYork-Presbyterian
NewYork-Presbyterian is one of the nation’s most comprehensive healthcare delivery networks, focused on providing innovative and compassionate care to patients in the New York metropolitan area and around the globe. In collaboration with two renowned medical school partners, Weill Cornell Medicine and Columbia University College of Physicians & Surgeons, NewYork-Presbyterian is consistently recognized as a leader in medical education, groundbreaking research and clinical innovation.
NewYork-Presbyterian has four major divisions: NewYork-Presbyterian Hospital is ranked #1 in the New York metropolitan area by U.S. News and World Report and repeatedly named to the magazine’s Honor Roll of best hospitals in the nation; NewYork-Presbyterian Regional Hospital Network is comprised of leading hospitals in and around New York and delivers high-quality care to patients throughout the region; NewYork-Presbyterian Physician Services connects medical experts with patients in their communities; and NewYork-Presbyterian Community and Population Health features the hospital’s ambulatory care network sites and operations, community care initiatives and healthcare quality programs, including NewYork Quality Care, established by NewYork-Presbyterian, Weill Cornell and Columbia.
NewYork-Presbyterian is one of the largest healthcare providers in the U.S. Each year, nearly 40,000 NewYork-Presbyterian professionals deliver exceptional care to more than 4 million patients.
For more information, visit www.nyp.org and find us on Facebook, Twitter and YouTube.
Weill Cornell Medicine
Weill Cornell Medicine is committed to excellence in patient care, scientific discovery and the education of future physicians in New York City and around the world. The doctors and scientists of Weill Cornell Medicine—faculty from Weill Cornell Medical College, Weill Cornell Graduate School of Medical Sciences, and Weill Cornell Physician Organization—are engaged in world-class clinical care and cutting-edge research that connect patients to the latest treatment innovations and prevention strategies. Located in the heart of the Upper East Side’s scientific corridor, Weill Cornell Medicine’s powerful network of collaborators extends to its parent university Cornell University; to Qatar, where Weill Cornell Medicine-Qatar offers a Cornell University medical degree; and to programs in Tanzania, Haiti, Brazil, Austria and Turkey. Weill Cornell Medicine faculty provide comprehensive patient care at NewYork-Presbyterian/Weill Cornell Medical Center, NewYork-Presbyterian Lower Manhattan Hospital and NewYork-Presbyterian Queens. Weill Cornell Medicine is also affiliated with Houston Methodist. For more information, visit weill.cornell.edu.