Precision medicine nets early results — with the promise of more to come
By Anne Machalinski
Portraits by John Abbott
In December 2013, right before she had her bladder removed, Irene Price heard the term “precision medicine” for the first time. She’d been diagnosed with cancer about five years earlier, and in the interim had exhausted the standard treatments—chemotherapy and a bladder-cancer-specific immunotherapy—both of which had shown early promise. But the oncecontained cancer continued to spread, and she was running out of time. Then her doctors told her they planned to analyze her tumor using next-generation DNA-sequencing technology to look for genetic alterations that might point to new treatment options. “I was excited,” says Price, a seventy-seven-year-old from Livingston, New Jersey, “and also anxious to see the results.”
When the tests came back, they revealed something unexpected: multiple copies, or amplification, of a gene typically associated with breast cancer growth. Based on these results, Price's medical team targeted the alteration using an approach not FDA-approved for her type of disease — chemotherapy with Taxol and Herceptin, drugs frequently used in breast cancer — and it worked. As of June 2015, the date of her most recent CAT scan, she has shown no evidence of cancer. "I don't think I'd be here without that test," Price says. Her oncologist agrees. "The genomic testing was extremely informative," says Dr. David Nanus, chief of the Division of Hematology and Medical Oncology. "I would never have used Herceptin without the precision medicine data."In December 2013, right before she had her bladder removed, Irene Price heard the term "precision medicine" for the first time. She'd been diagnosed with cancer about five years earlier, and in the interim had exhausted the standard treatments — chemotherapy and a bladder-cancer-specific immunotherapy — both of which had shown early promise. But the once-contained cancer continued to spread, and she was running out of time. Then her doctors told her they planned to analyze her tumor using next-generation DNA-sequencing technology to look for genetic alterations that might point to new treatment options. "I was excited," says Price, a 77-year-old from Livingston, N.J., "and also anxious to see the results."

Caring relationship: Dr. David Nanus with patient Irene Price
While Price represents an early ideal of this approach, patient success stories are expected to multiply as clinicians increasingly target the genomic characteristics of a disease rather than its site of origin. Having already performed comprehensive genomic testing on about 300 patients with advanced cancers over more than two years, the Caryl and Israel Englander Institute for Precision Medicine at Weill Cornell is poised to lead the charge toward making this personalized approach the standard of care. The institute received a generous gift from the Englander family this month to widen its mission to emphasize dermatological malignancies as well as metabolic diseases, cardiovascular disease, genetic disorders, and respiratory diseases and eventually offer precision medicine to as many as 6,000 cancer patients a year. "There's no playbook. We need to establish our own guidelines in real time," says the institute's founding director, Dr. Mark Rubin, the Homer T. Hirst III Professor of Oncology in Pathology. "Our goal is simply to direct the patient to the right care. It's really out-of-the-box thinking, and we're innovating and figuring out what to do all the time."
While the Englander Institute — comprising a growing team of about 50 — offers advanced-stage cancer patients access to the most powerful genomic test in New York State, three years ago the organization didn't even exist. Dr. Rubin is a renowned pathologist and expert in genomics research who has dedicated his career to understanding and combating prostate cancer; he came to Weill Cornell seven years ago to develop a genomics center. Since the early days of his career — which has included work at a number of prestigious institutions like NewYork-Presbyterian/Columbia University Medical Center, the University of Michigan, and Harvard Medical School — he has always believed in the concept that doctors are "clever detectives," making discoveries about their patients based on direct observations and clinical data. "Adding genomics to the toolbox means having thousands of additional pieces of information that can help you make discoveries and answer questions," he says. "That's the genius of medicine."
Guided by his vision, the Institute for Precision Medicine opened in January 2013 as one of the first entities of its kind. Dr. Rubin's first order of business was to gather a dream team of clinicians, researchers, data experts, and others. Its leadership includes Dr. Himisha Beltran, a medical oncologist and physician-scientist who serves as the Englander Institute's director of clinical activities, and Dr. Olivier Elemento, who joined the team from the HRH Prince Alwaleed Bin Talal Bin Abdulaziz Alsaud Institute for Computational Biomedicine (ICB) at Weill Cornell, where he heads the Laboratory for Cancer Systems Biology.
Although Dr. Rubin's team has advocated for precision medicine's potential for years, the approach got widespread attention in January when President Barack Obama used his State of the Union address to announce a national initiative, which earmarked $215 million from the proposed 2016 budget for expanded clinical and research development in the field. More recently, the National Cancer Institute launched a nationwide precision medicine research study that will sort patients into treatment groups based on genomic alterations in their tumors, representing a sea change in oncology. "All of a sudden, there's an environment where people want to know what precision medicine is and what's going on," says Dr. Rubin, who was invited to the Obama initiative's announcement at the White House. "The fact that we are already doing it allows us to drive the conversation and innovation in this field."
A Key Test

Dr. Mark Rubin
To pinpoint individualized therapies, a number of clinicians offer patients a simple genomic test that canvasses a tumor tissue sample for mutations on 50 to 400 genes that can be targeted with proven therapies. This process is called "focused" or "panel" sequencing. But there's a second, more comprehensive type of test that reviews up to a hundred times more genes to uncover alterations basic tests can miss. Called "whole exome" sequencing, this process looks where DNA is transcribed into RNA — a region of the gene called the exome — and helps researchers find new therapeutic pathways for patients who have exhausted standard protocols. While some area medical centers offer the first kind of test, Weill Cornell is the only institution in New York State to offer the second.
The Englander Institute-developed test, called EXaCT-1 (for EXome Cancer Test-1) was recently described in JAMA Oncology by Dr. Beltran and colleagues; it looks for any and all mutations across more than 21,000 genes. Because it doesn't focus on expected mutations or those tied to a specific treatment protocol (what physicians call "actionable"), it's especially effective in pinpointing previously undetected alterations in patients' tumors. (As Dr. Elemento notes: "The advantage of sequencing the entire tumor genome is that we're not going to miss anything.")
Early on, the team decided to focus on patients with advanced-stage cancers. "The typical approach in cancer treatment is that you first start with the most advanced disease, because those patients are out of options," Dr. Rubin explains. "The discovery of an effective drug in this advanced setting paves the way for using it earlier and earlier with the hope that it will prevent the tumor from progressing. That's where we can have a cure."
Dr. Rubin and his team emphasize that the test is not just about sequencing a tumor's DNA. It's about gathering that data — enough to fill a 100-gigabyte hard drive — and then synthesizing it and delivering the results in a way that's easy for the physician to access, read, understand and share. "How the clinician interacts with the data and communicates the findings back to their patient is the most important thing," Dr. Rubin says.
The process — which requires extensive teamwork between surgeons, medical oncologists, and radiation therapy experts — starts with the samples: the patient's blood (the control) and tumor tissue, ideally with a large percentage of abnormal cells. DNA sequencing takes about two days, after which the raw data is sent to a super-computer for additional, automated analysis. At the end of this process, an easy-to-read report — including clinical information, images of the tumor, and a summary of discovered mutations — is generated. Someday soon, Dr. Rubin hopes to benefit patient s by seamlessly delivering such reports directly to a clinician's iPad, Android device, or Apple Watch — "the simplest solution in an elegant, easy way" — but today, it's e-mailed as a PDF file. On it, the list of mutations is grouped into three categories. At the top are mutations that drive cancer growth and are connected to a known treatment protocol; the middle have been previously observed in other tumors and may very well drive the disease but are not well understood and not therapeutically targetable; and the bottom are of unknown significance, and not yet connected to cancer.
On Fridays, the precision medicine team meets in a tumor board to review the results of one to five patients to discuss available treatment options and next steps. From start to finish, the process of running the sequencing test, generating the report, reviewing the findings, and pursuing new treatments currently takes four to six weeks — slower than a focused test that looks at only a few hundred genes, but still fast enough to influence outcome if there's a new therapeutic option available. Dr. Rubin, though, hopes to speed up that timeline to two weeks from the start of the test to the treatment recommendation.
Big Promise, Mixed Results
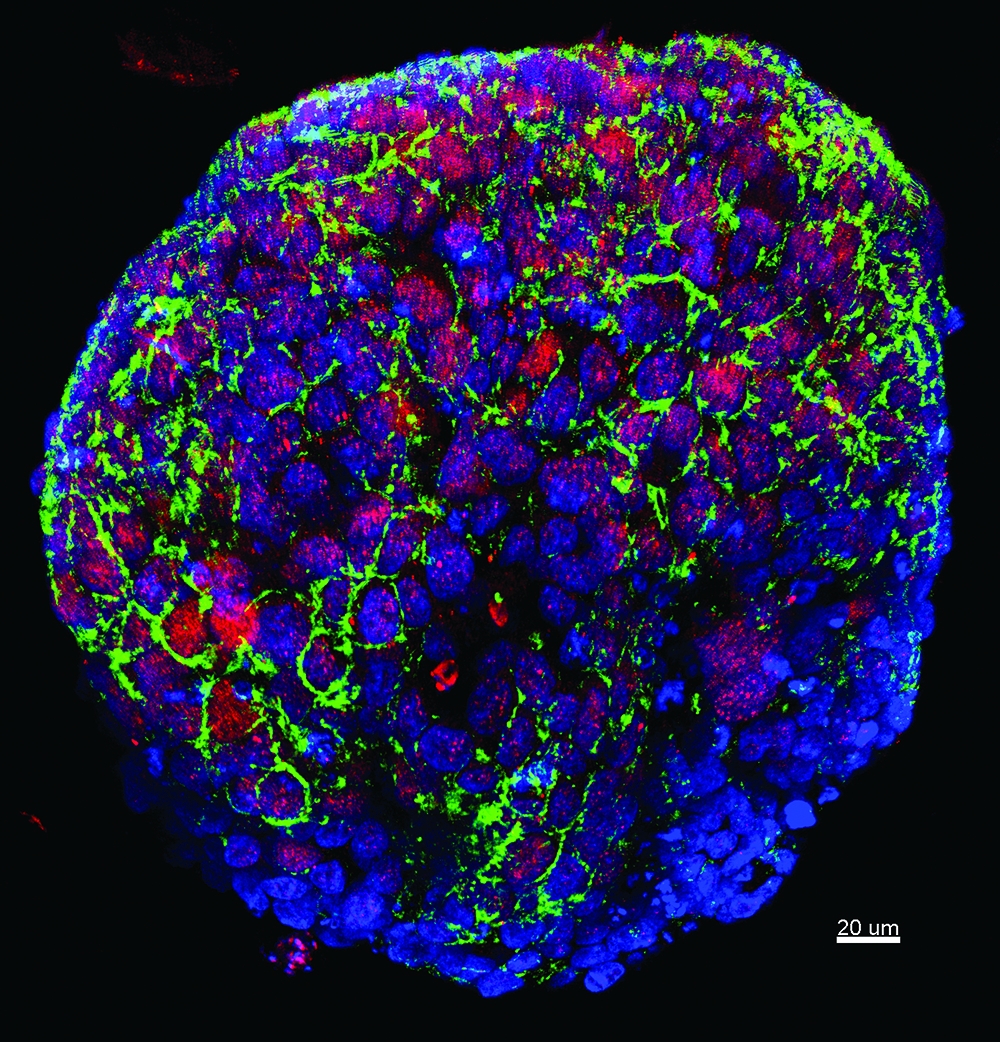
Genomic testing: Tumor cells, above and image below, from a biopsy of a patient enrolled in a trial evaluating the efficacy of whole exome sequencing. Image credit, above and below: Dr. Loredana Luca
For Irene Price, the standard treatment — flushing out her bladder with a weakened form of a tuberculosis vaccine pathogen, called BCG therapy, and monitoring her disease — was enough to keep the cancer contained within her bladder for years. After it spread to her lymph nodes, Price underwent chemotherapy, and in October 2013 she was given a clean bill of health. But the cancer returned within weeks. That December, she had her bladder removed.
Early in 2014, tumor tissue from that surgery was sequenced and the precision medicine report came back with a targetable alteration: over-expression of a gene called HER2. Amplification of this gene is known to drive cancer growth, and it is frequently detected in breast cancer patients, says Dr. Nanus, who is also the Mark W. Pasmantier Professor of Hematology and Oncology in Medicine and the associate director of clinical services at the Sandra and Edward Meyer Cancer Center at Weill Cornell and NewYork-Presbyterian Hospital. An antibody-based therapy that targets that alteration has saved many lives, but it is not normally used in a patient like Price, who had bladder — not breast — cancer. But because Price had run out of options, and her cancer had spread to her liver — giving her, statistically, about a year to live — they gave it a try. And it worked. "I can tell you very clearly that we would not have been able to find that mutation using a 50-gene panel," says Dr. Elemento, noting that commonly used focused panels do not look for gene amplifications or deletions.
But not all precision medicine results are as straightforward as Price's. While her report included a known alteration that had a highly proven and available therapy connected to it, many patients' reports don't reveal mutations that can be tackled with readily accessible treatments. New research that Dr. Beltran, Dr. Elemento, Dr. Rubin, and colleagues recently published in JAMA Oncology detailed the Englander Institute's work with its first 97 patients. They explained how they were able to pinpoint previously unknown mutations and recommend new therapeutic options 92 percent of the time — but only 5 percent of those patients actually gained access to the recommended treatment. In some cases there wasn't a clinical trial being offered nearby. In others, clinicians couldn't access the proposed drugs, or they were too expensive. "As trials are developing and the medications become more accessible, I think that success rate will change in the coming years," Dr. Beltran says. But for now, she says, accessibility of therapies is one of precision medicine's major limitations.
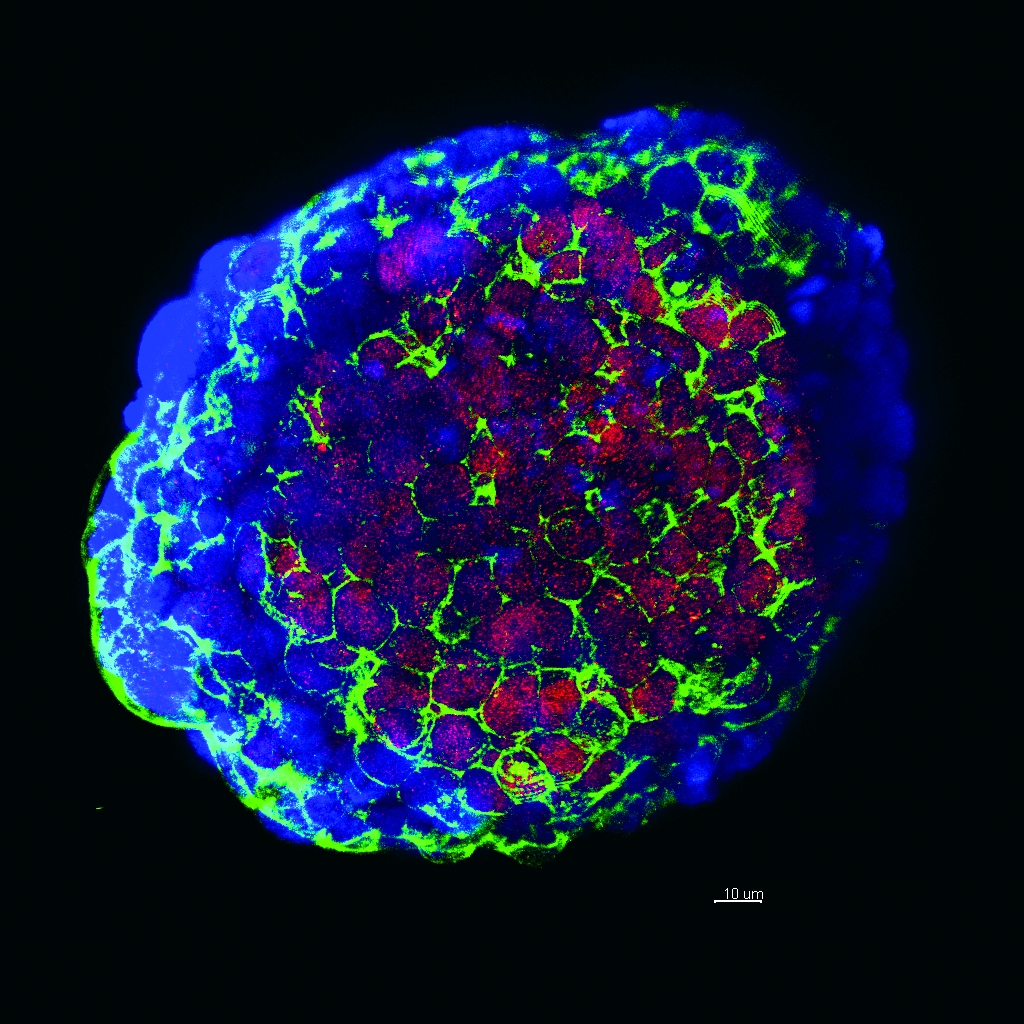
Even in Price's case, once the clinicians found a target, the recommended treatment wasn't FDA-approved for bladder cancer, so Dr. Nanus and others from the team had to petition her insurance company to pay for it. They gained that approval, but advocating for each and every precision medicine patient — or starting single-subject clinical trials to get them the drugs that they need — is time-consuming and unsustainable. "We need to figure out a process to quickly and seamlessly get FDA approval and move past this bottleneck," Dr. Rubin says.
Another challenge of using the EXaCT-1 test — which reviews genes without looking for specific mutations, like panel tests do — is that the majority of alterations fall into the category of "unknown significance." This is why research is so important, Dr. Beltran says. Some of those alterations are likely driving cancer growth and spread — and by identifying these key drivers and understanding how they work, scientists can develop new therapeutic options — but many others are likely inconsequential to the disease. "We're learning from each individual patient," Dr. Beltran says. "By studying the extremes — people who respond really well and people who don't respond at all when they should — we are learning more about which molecular alterations are important in predicting response to treatment."
To learn more about mutations, the precision medicine team often splits the biopsied tumor tissue in half. (Indeed, a patient can only benefit from precision medicine when his or her cancerous tissue is made available for analysis.) While one part goes toward DNA sequencing, the other is used in the lab to grow mini-tumors (also called organoids) or implanted into mice. These tumors can be propagated indefinitely and treated with various drug combinations in a search for the most effective treatment strategy. In time, the team hopes to identify new targetable mutations and develop treatment protocols based on this bench work.
The Case for Data
While gathering genomic information can help clinicians determine the right treatment for each patient, the development of robust, searchable databases (a process that received early attention from the ICB) is also vital to precision medicine's success. Currently, the Englander Institute has its own internal database, which it updates with information on genetic mutations, associated drug trials, and patient outcomes. But that data set, while growing, is relatively small. Creating databases that incorporate clinical information from patients at institutions across the country and around the world is essential, Dr. Elemento says. "Individual institutions just can't keep up," he says, "and it doesn't make sense for everyone to have their own database." Dr. Rubin agrees. "With vast clinical data, if we have a patient sitting in front of us who has a mutation that we've never seen before, we can ask the question, 'Has anyone ever seen it before?'" he says. "One of the biggest hurdles within precision medicine is to marry clinical and genomic data and also have a way to share that data. Our dream is to find a way to do this."
The New York City Clinical Data Research Network (NYC-CDRN), launched in January 2014, is an early example of effective clinical data sharing to support research. Notable for its breadth and scope, it includes the largest and most diverse collection of patient records in the country, connecting more than 40 million encounters from the medical records of a growing pool of more than 4 million individual patients records from six healthcare systems in New York City. "For common conditions, getting broad data like this is phenomenal," says the project's principal investigator, Dr. Rainu Kaushal, chair of the Department of Healthcare Policy and Research and a national leader in developing medical data systems. "For rare diseases or mutations, it's even more valuable because you can get a significant sample size to study them."
Investigators working on a wide variety of diseases and conditions are already requesting access to the network's data, which could eventually include everything from the results of an annual physical to those of a genomic test, Dr. Kaushal says. Doctors, too, will be able to gain valuable insights from it; for instance, by tracking how cancer patients with a rare mutation have responded to a certain treatment, they can make more informed decisions in treating people with that same genetic make-up.
Dr. Beltran notes that while the identity of individual patients is scrubbed from these databases, it's important that there be a way to re-identify them, if necessary. "I think in the next five years we're going to be able to present patients who have genomic information in their medical records with new findings and treatment options," she says. "If a mutation moves from the 'unknown' to the 'actionable' category, there would be good reason to follow up with patients who have it."
Looking Ahead
While at present the precision medicine approach is primarily used in cancer diagnosis and care, experts agree that in the future it will have wider clinical relevance. As our understanding of human biology improves, Dr. Elemento says, doctors will increasingly look to analyses of "germline" — or heritable — DNA, for alterations that predispose people to Alzheimer's, schizophrenia, pulmonary disease, or other conditions. BRCA1 and BRCA2 mutations — germline DNA variations connected to an increased risk of developing breast or ovarian cancer — are the most prominent examples of how this type of test is used to date.
Increased use of sequencing tests in cancer is likely in the future, too, Dr. Rubin says, especially earlier or more often in the diagnosis and treatment stages. The results may dictate not only how a patient is treated, but also what approaches are used early on, like whether to try the latest immunologic therapies. "I think a year or two from now — but not five — insurance companies will be advocating for patients to have some sort of genomic test before they're approved for treatment," he says.
\But to make this type of test more accessible, its costs will have to decrease. While EXaCT- 1, which is currently being reviewed by the New York State Department of Health for clinical use, currently costs a few thousand dollars per patient on paper, it's likely closer to $30,000 if everyone's time and effort is taken into account, Dr. Rubin says. But that doesn't mean the test — or approach — should be abandoned. "It's like if you were building a prototype car," he says. "The first car might cost $3 million; you would never sell it. But you can't get to the final car that costs $15,000 or $20,000 until you've done all the testing and established a production line. Soon, all of these worries about the turnaround time and cost are going to be trivial." Dr. Nanus agrees, comparing genomic testing's rise in technological sophistication and drop in cost to cell phones, which were once expensive but are now affordable and ubiquitous. "Someday, it will be the standard of care," he says. "Everybody will get their tumor sequenced."
Despite the high cost and mixed results, Dr. Rubin says, there's substantial value in charting new territory in this field. By being in the vanguard, he says, the Englander Institute for Precision Medicine can build things like the EXaCT-1 test from the ground up to its own specifications. He compares the Englander Institute to a start-up company that helps define an industry. "We could wait and then re-enter this arena when there's a kit and the test will be done in an hour," Dr. Rubin says. "But with contributions from pathology, oncology, urology, and computational biology, we're gaining expertise, we're honing our communication platform so that the results make sense to the patient, and we're conducting meaningful research that's going to have a long-lasting impact."
For Price — who still has CAT scans and heart tests every few months, and regularly checks herself for new lumps in her groin, where they appeared in the past — the glass is more than half full. Precision medicine and her Weill Cornell team have bought her more time with her family — allowing her to see the birth of her second great-grandchild, Hailey, in March; to help move her granddaughter to a town just 35 minutes away; and to gather with her extended family to celebrate her grandson's kindergarten graduation this summer. Despite being on maintenance chemotherapy — a Herceptin drip every three weeks for 30 minutes — she's feeling good. "I'm just so thankful," Price says. "I'm not ready to go."
This story first appeared in Weill Cornell Medicine,Vol. 14, No. 2.

